重金属在煤气化过程中迁移规律及在气化渣中浸出行为和固化处理综述
0 引 言
根据我国统计局发布的最新能源消费报告,现阶段煤炭消费在我国能源结构中仍占据主导地位[1-2]。然而传统煤电技术运用场景单一,制约煤炭清洁高效利用[3]。煤气化技术可以弥补煤电技术不足,为合成煤基化学品、制备气/液化燃料、IGCC发电等工业提供基础,减少对国外石油(70%)、天然气(42.8%)的高度依赖,提高煤的高效清洁利用,是今后主流发展技术之一[4-7]。
煤气化渣(Coal Gasification Slag,CGS)是原煤和气化剂在气化过程中产生的固体废物(副产品),通常分为粗渣和细渣[8-9]。其中,粗渣是指顺着底流从气化炉灰渣漏斗排出的大颗粒灰渣;细渣是指以飞灰形式随合成气排出,经洗涤系统和旋风分离器等分离系统收集得到的固体沉积物[10-11]。据统计,我国煤气化行业每年排放的煤气化渣量超3 300万t,且总量不断增加[5]。目前,填埋和堆存是主要的灰渣处置方式,灰渣利用价值极低[4,12]。为此,大量学者对其进行高附加值产品转换探索,如路基、建材、陶瓷、分子筛、催化剂、土壤或水体修复剂等,取得一定成效[13-17]。
然而,煤气化渣含有汞(Hg)、铬(Cr)、镉(Cd)、铅(Pb)、锌(Zn)、镍(Ni)等有毒重金属,未经处理可能会导致有毒重金属浸出,对生态环境和人体健康造成一定危害[18-19]。JIANG等[19]使用四步逐级化学提取法测定了煤气化渣中重金属的化学形态,发现细渣中Pb、Zn、Ni等重金属具有较高的淋滤系数,会在环境中浸出。GUO等[20]研究了气化灰渣对环境影响,表明底灰中的重金属Cr以填埋方式处理会释放到环境中。因此,煤气化渣中重金属,不仅制约其综合利用、降低商业价值,还会引起一系列环境污染问题[14]。
为解决上述问题,国内外在煤气化过程中重金属释放迁移、浸出行为、固化处理等方面进行了大量研究。重金属在煤气化过程中释放迁移受气化温度、气化气氛、气化压力等多种因素影响[21-23]。一般认为,气化温度越高、气氛还原性越强,越有利于重金属释放迁移[24-26]。煤气化渣中重金属浸出行为受到浸出液pH、温度、固液比、灰渣粒径等多种因素影响,其中pH是主要因素[27-30]。灰渣中重金属的浸出率与毒性直接相关,可交换的赋存形态对环境危害更大[31-32]。为降低灰渣中重金属可交换态的含量,减少对环境污染,学者们通过水泥固化、热处理固化、水热处理固化等固化技术将重金属的可交换态转化为不溶态,将重金属固定在灰渣中,达到脱毒目的[33-35]。
因此,煤气化渣中的重金属是影响灰渣资源化、高附加值应用的直接因素。笔者总结了6种有毒重金属(Hg、Cr、Cd、Pb、Zn、Ni)在煤气化过程中的迁移规律和影响因素、气化灰渣中赋存形态和浸出行为、气化灰渣的固化和处理技术等,为煤气化渣的综合利用提供理论依据。
1 重金属在煤气化过程中迁移规律
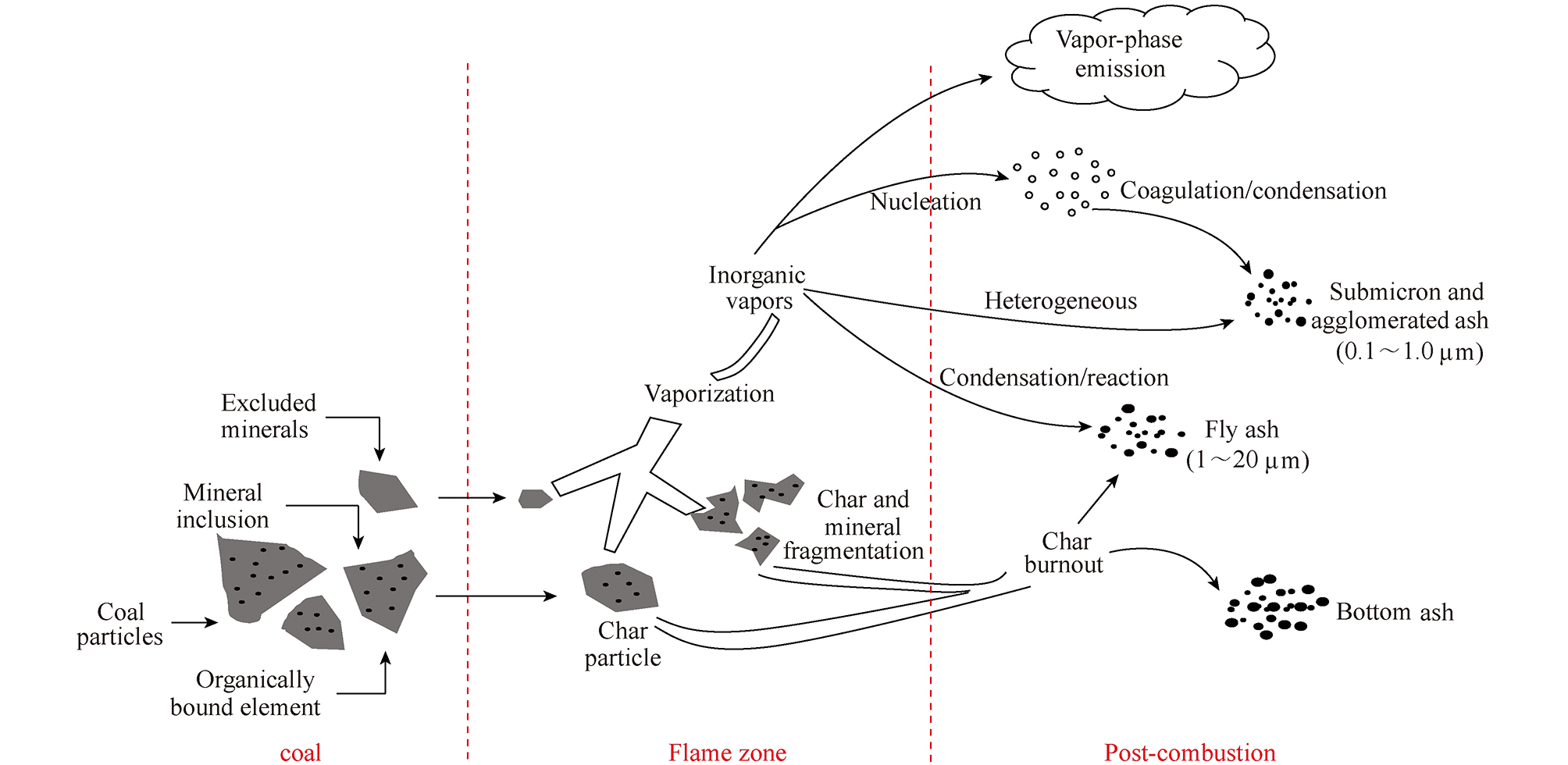
CLARKE[36]根据煤中痕量元素(质量分数小于0.01%)挥发性差异和在产物中的分布情况,将其分为3类:第1类为难挥发元素,这类元素主要富集于残渣中;第2类为较易挥发性元素,这类元素挥发至烟气中并通过均相成核作用和非均相凝结形式冷凝富集在飞灰表面;第3类为极易挥发元素,这类元素在反应过程中大部分发生迁移,一般以气相形式存在,仅有少部分通过凝结残留在固相中。本文涉及Hg、Cr、Cd、Pb、Zn和Ni等元素均属于煤中痕量元素。
1.1 汞(Hg)
Hg在煤气化过程中属于极易挥发元素,大部分转移至煤气和细渣中[37]。Hg在煤气化过程中的释放分为3种类型:元素汞(Hg0)、氧化汞(Hg2 )和颗粒汞(Hgp)[38]。其中,Hg0(g)易挥发,是主要的气相形态,其在水中溶解度有限,在大气中停留时间长;Hg2 (g)则极易溶于水,可通过湿法烟气净化系统去除;Hgp可通过除尘器去除,不易进入大气中[39]。余琛[40]研究煤热解过程中汞释放和形态转化规律,表明热解温度达到800 ℃时,煤中80%以上的汞释放至烟气中。同时,WU等[41]通过热力平衡计算,发现大部分Hg元素在温度超过800 ℃时以气态单质形式挥发至煤气中,这也与余琛和HELBLE的试验结果一致[42]。BUNT等[43]和根据热力学平衡计算并预测了Hg在固定床气化炉在干燥区、热解区和还原区中反应平衡特点:汞在干燥区迅速挥发,随后在气化炉的快速热解区内进一步分配到气相中;几乎所有的汞在热解区末端都变成了气相,即热解结束时(725 ℃),化合态结合的Hg(如HgSe(g))消失,只剩下元素 Hg(g);而还原区中的Hg释放几乎无变化,如图1所示。

图1 Hg在煤气化过程中分布特征[43]
Fig.1 Distribution characteristics of Hg during the coal
gasification process[43]
Hg在煤气化过程中释放迁移受气化温度、反应气氛和煤种等因素影响。其中,温度是影响Hg挥发程度的最重要因素。一般认为,随着气化温度升高,Hg的挥发程度越高,在煤气和细渣中富集程度增加[21]。此外,也有学者发现,高气化温度下底灰中仍检测到少部分Hg,其原因是煤中S和Hg发生反应生成HgS或HgSO4被固定在灰渣中,且气化渣中Hg和S呈正相关[44]。值得注意的是,不同煤种之间略有差异,王云鹤等[45]对比大同煤和神府煤气化时重金属迁移规律,发现2种煤气化中Hg释放率存在一定差别。相较于氧化氛围,还原气氛可以增加Hg的挥发释放量[22]。此外,在新型煤气化工艺中,载氧体是煤和气化剂反应的重要桥梁[46]。大部分载氧体为第四周期过渡金属如铁(Fe)、锰(Mn)或铜(Cu)等,这些过渡金属元素对Hg的氧化和吸附有一定影响[47-48]。AN等[49]研究了载氧体(CuFe2O4)对Hg的释放影响,发现载氧体通过吸收Hg(g)的方式促进Hg(g)向Hgp转化以及Hg0向Hg2 转化。
1.2 铬(Cr)
Cr在煤气化过程中属于难挥发元素[50]。FRANDSEN等[51]认为,Cr2O3(s)是标准还原条件下的稳定存在形式。在气化过程中,Cr会被高岭土、铝土矿、石灰等矿物固定在底灰中[30],且相较于原煤,气化灰渣中Cr浓度明显高于原煤[52]。李玉兰等[53]认为即使热解温度达到1 600 ℃,大部分Cr元素仍保留在灰渣中。在煤气化渣中,Cr的主要形态是与铝硅酸盐结合存在的残余不溶态[54],其原因是煤气化灰中矿物主要由硅铝酸盐组成,Si—O四面体是其基本的结构单元,Al的行为与Si相似,表现出四重配位,形成多种Al—Si配位结构,而Cr在气化灰渣中的作用类似于Al,导致Cr3 吸收并固定到非晶硅铝酸盐网络内[55]。BUNT等[50]根据热力学平衡计算并预测了Cr在固定床气化炉在干燥区、热解区和还原区中反应平衡特点:干燥区、热解区和还原区内的Cr含量差异显著,Cr含量随气化炉床层深度而大幅增加;在干燥区和还原区内,Cr含量分布发生反转,可能由于Cr氧化态的改变导致部分挥发,如图2所示。

图2 Cr在煤气化过程中分布特征[50]
Fig.2 Distribution characteristics of Cr during the coal
gasification process[50]
即使Cr属于难挥发元素,其在煤气化过程中释放迁移仍受反应条件影响。Cr的释放率随气化温度升高而增加,但提高空煤比将减少Cr释放,有利于Cr在气化灰渣中富集[21-22]。BARTONOVA等[56]通过热力平衡计算预测Cr在高温下的行为,表明还原气氛更有利于Cr在灰渣中固定。此外,气化气氛还会影响灰渣中Cr的形态转化,空气中加热煤粉会促进Cr6 的形成,并提高煤灰中Cr浸出率;而N2或 H2气氛下加热煤粉有利于降低Cr6 浓度(灰渣中的Cr浸出率降低了100倍)[57]。
1.3 镉(Cd)
Cd在煤气化反应过程中属于半挥发元素,其表现为在高温气化过程中挥发,又随温度下降冷却至细渣中[45]。煤中Cd元素主要与黄铁矿、闪锌矿等矿物质伴生,部分赋存在其他难溶矿物质中[58]。在热解和气化过程中,Cd的释放率在30%~90%[21,43,59]。CHEN等[60]研究煤气化过程中无机元素释放行为,表明与煤有机组分或含硫矿物相结合的Cd随着这些组分的分解而释放出来,而与硅铝酸盐矿物稳定结合的Cd则被保留在半焦中。BUNT等[43]根据热力学平衡计算并预测了Cd在固定床气化炉在干燥区、热解区和还原区中反应平衡特点:仅有12%的Cd在干燥区挥发;热解结束时,45%的Cd已挥发至气相;而还原区与热解区的结果相比,Cd含量明显降低38%;只有13%的Cd留在固定床气化炉的灰渣中。Cd在煤气化过程中的分布特征如图3所示。

图3 Cd在煤气化过程中分布特征[43]
Fig.3 Distribution characteristics of Cd during the coal
gasification process[43]
气化产物中Cd分布受气化温度、反应气氛和煤种影响,气化温度是主要影响因素。热解温度在800 ℃时,与有机和硫化物结合的Cd挥发释放,而残渣态中Cd含量在半焦中比例为80%~90%;热解温度超过800 ℃,Cd挥发性增强,以硫化镉(CdS)形式释放,使Cd在焦油中含量增加,在气化灰中含量减少;剩余Cd会与灰渣中硅铝酸盐反应并被固定在底灰中[45,61]。气化温度高于900 ℃,Cd富集在布袋低温焦中,且相较于高温焦,Cd在低温焦中相对富集系数较高[21]。其他条件对Cd在产物中的分布影响较小,随空煤比增加,Cd在高温焦中的相对富集系数略增加,而在低温焦中相对富集系数略降低,但Cd在低温焦中的相对富集系数仍明显高于高温焦[22];随气化压力增大,Cd在低温焦中的相对富集系数呈减小趋势,而底渣中的相对富集系数呈增加趋势[23]。此外,Cd在气化灰渣中的浓度随灰分粒径的增加而降低[62]。
1.4 铅(Pb)
Pb在气化过程中属于挥发性元素。煤中Pb的赋存形态主要伴生于方铅矿和黄铁矿,与硫化矿物表现出无机亲和力[60,63],这使得Pb在煤气化过程中具有较高的迁移率[64]。在气化过程中,Pb最有可能以PbS、PbSO4和PbCl2的形式气化释放至气相[43,65]。前2种化合物可作为可氧化态提取,而后一种化合物可与Al2O3和SiO2反应并固定在铝硅酸盐晶格中,作为残余不溶态[66]。THOMPSON等[67]使用热力学模拟预测煤气化过程中微量元素的平衡分布,发现Pb在气化过程中几乎完全释放迁移,表明Pb更倾向于高温蒸发释放至煤气中,然后与煤气共同冷却,上述模拟结果与试验结果一致[42]。BUNT等[43]根据热力学平衡计算并预测了Pb在固定床气化炉干燥区、热解区和还原区中反应平衡特点:煤中75%的Pb在干燥区挥发;在热解结束时,几乎所有Pb挥发至气相中;在还原区内,Pb分配减少至70%,这可能是由于Pb氧化态发生了变化,如图4所示。

图4 Pb在煤气化过程中分布特征[43]
Fig.4 Distribution characteristics of Pb during the coal
gasification process[43]
根据BUNT等[43]研究结果,Pb在气化炉中容易释放,气化温度达到900 ℃时很难在底灰中检测到,且Pb会以硫化汞(PbS)的形式存在于高温煤气,然后随着煤气冷却在气化渣颗粒上析出。因此,气化过程中Pb主要受温度影响,随气化温度升高,Pb释放率增加,在底灰中富集系数减小[21]。同时,H2气氛有利于促进Pb的释放[68];增加气化压力,则有利于Pb在底灰中富集,减少低温焦中的富集[23]。因此,在工业气化炉中,Pb更易富集在气化细渣中,并随气化细渣粒径的增加而增加。此外,Pb的高挥发性导致其在煤气化渣中的稳定性降低[69]。
1.5 锌(Zn)
Zn在煤气化过程中属于挥发性元素。根据FRANDSEN等[51]研究结果,Zn(s)在还原条件下的蒸发点为575 ℃,725 ℃以上,仅存在气态Zn(g)。对于低硫煤的气化过程,气化温度在625~725 ℃时,产物中Zn的形态为硫化锌(ZnS)、Zn(g)及少量ZnCl2(g);气化温度高于825 ℃时,Zn(g)是主要存在的含锌化学物质[70]。CHEN等[71]报道了Zn在气化渣中分布,表明Zn主要分布在可氧化态、可还原态和不可溶态。BUNT等[72]根据热力学平衡计算并预测了Zn在固定床气化炉在干燥区、热解区和还原区中反应平衡特点:在干燥和热解区,原料煤中的Zn以闪锌矿(ZnS)的形式保持稳定;温度超过625 ℃,ZnS分解后在725 ℃形成Zn(g);温度超过825 ℃,计算平衡曲线预测在反应器的还原区内,气化系统中所有Zn以Zn(g)形式存在,并作为稳定相保持恒定,直到还原过程结束,如图5所示。

图5 Zn在煤气化过程中分布特征[72]
Fig.5 Distribution characteristics of Zn during the coal
gasification process[72]
气化产物中Zn的分布主要受气化温度影响,且Zn的释放率随气化温度升高而增加[70]。在气化细渣中,高达32.73%的Zn与碳酸盐有关,表明Zn在灰渣中赋存方式可能与碳酸盐相结合[30]。灰渣顺序化学提取显示灰渣中66%~71%的Zn以酸溶形态、可还原形态和可氧化形态存在,进一步说明Zn在气化过程中迁移至煤气中的倾向更高[73]。此外,硅酸盐中的部分Zn在高温下与Al2O3和SiO2反应形成 ZnAl2O4和Zn2SiO4,这是残留不溶态的来源[74]。
1.6 镍(Ni)
Ni在煤气化过程中属于半挥发性元素。Ni在煤中与黄铁矿共存,在气化过程中首先形成Fe-Ni-S矿物,随后转化为NiS[75-77]。NIU等[78]研究Lurgi气化炉中微量元素释放行为,发现Ni在气化渣中明显富集,且Ni在细渣中含量高于粗渣,表明Ni在气化过程中的释放行为是挥发-冷凝机制,在高温区域挥发至气相,在低温区域冷凝并吸附于细颗粒表面[79]。FRANDSEN等[80]在标准还原条件对Ni的稳定形式进行研究,发现Ni在145 ℃以内以NiS2(s)形式存在;145~275 ℃,Ni以NiS0.84(s)形式存在;275~770 ℃,Ni3S2(s)是Ni的稳定形式;770 ℃后,Ni(s)开始出现;1 425 ℃以上,系统中仅存在Ni(g)和少量NiO(g),这与MA等[77]热力平衡计算结果一致。BUNT等[72]根据热力学平衡计算并预测Ni在固定床气化炉在干燥区、热解区和还原区中反应平衡特点:Ni在反应器系统的干燥和热解区内的固相中保持稳定,温度高达725 ℃;在还原区内,Ni开始挥发至气相中,在1 225 ℃时挥发量为10%,在1 316 ℃时挥发量为100%,如图6所示。

图6 Ni在煤气化过程中分布特征[72]
Fig.6 Distribution characteristics of Ni during the coal
gasification process[72]
Ni在煤气化过程中释放迁移受气化温度、反应气氛和气化压力等因素影响,其中温度是主要影响因素。随气化温度升高,Ni释放率增加,底渣中Ni的相对富集系数逐渐减小,低温焦和高温焦中Ni的相对富集系数逐渐增加[21]。随着空煤比增加,Ni在高温焦中相对富集系数略增加[22]。随气化压力增大,Ni在气化渣中相对富集程度呈略增大趋势[23]。此外,煤气化渣中Ni的富集程度随灰渣粒径增加而降低,其原因为细小灰渣中存在具有较强吸附能力的多孔碳,会捕获煤气中Ni等重金属[78]。值得注意的是,灰渣中Ni总量有时存在超平衡现象,即超过输入煤中Ni总量,这归因于气化组件腐蚀引起灰渣中Ni含量增加[81]。
2 重金属在气化灰渣中浸出行为
煤中有毒重金属在气化过程中转移至灰渣,对气化渣安全处置、综合利用和生态环境带来挑战。了解气化渣中重金属浸出行为和影响因素将为重金属环境污染风险控制提供必要的理论依据。煤气化渣中重金属在浸出液中的浸出行为受浸出液pH、灰渣粒径、固液比、浸出时间、浸出温度和重金属的赋存形态等因素影响。浸出液的pH是影响灰渣中重金属浸出行为的主要因素[82]。同时,提高浸出液温度有利于提高灰渣中重金属的溶解速率和浸出扩散[83]。此外,重金属浸出动力学为理解不同类型气化灰渣中重金属的溶解和浸出机理提供必要的分析方法[84-85]。
2.1 汞(Hg)
Hg属于易挥发性元素,在经历高温气化反应后大部分迁移进入气相中,仅有1%左右的Hg留在灰渣中,其平均相对富集系数为0.004,远小于1[86]。Hg在气化灰渣中的总量远低于粉煤灰,且细渣中Hg的富集程度高于粗渣。Hg总浓度与可浸出Hg之间始终缺乏相关性,固液比或浸出时间等对Hg浸出没有显著差异[87]。灰渣中Hg的浸出可能受水相吸附影响,吸附的Hg分子之间相互作用强烈[88]。总体而言,气化灰渣中Hg的浸出浓度低于其他重金属浓度,远低于GB 5085.3—2007《危险废物鉴别标准:浸出毒性鉴别》中规定的限值,表现出无毒性[89]。
2.2 铬(Gr)
Cr3 和Cr6 的存在不仅对煤气化渣的毒性至关重要,也影响灰渣中Cr的迁移率。一般来说,灰渣中Cr6 比Cr3 更易从固体燃烧副产物中浸出[57]。气化灰渣中 Cr 的主要价态是Cr3 ,有些灰渣中甚至不含Cr6 ,因此Cr3 是灰渣中的主要形态。由于大部分Cr3 与硅铝酸盐缔合(存在玻璃相中),使溶解性差,对浸出行为和毒性产生积极影响[90]。这种情况下,气化灰渣中Cr3 只有在非常低pH和还原条件[91]下才能浸出,且Cr浸出率随渗滤液酸度的增加而增加[92]。酸浸可有效去除气化灰渣中Cr3 (去除率高达97.93%)[93]。Cr的浸出效率[94]如图7所示,Cr的浸出行为随酸浓度变化存在一个突变,并受固液比和时间影响,温度对浸出效率影响不大。GUO等[18]建立了Cr浸出动力学模型,发现Cr的浸出活化能较低,这也意味着灰渣中Cr的形态可能包括一定量的酸溶态和可交换态,降低浸出液pH有利于灰渣中Cr的浸出。

图7 不同条件下Cr的浸出效率[94]
Fig.7 Leaching efficiency of Cr under different conditions[94]
2.3 镉(Cd)
Cd的浸出行为[92]如图8所示。当浸出介质为酸性时,Cd的浸出率由不溶变为中溶[92]。Cd酸性条件下呈现较高可浸出性和流动性[95],其浸出率随浸出液酸度增加而增加[92],随灰渣粒径增加而减小[29]。然而,醋酸的酸浓度(1~5 mol/L)对Cd的浸出行为没有影响,归因于灰渣中铁化合物对Cd有吸附作用,并抑制了其在较低浓度的醋酸溶液下浸出[27]。LANGE等[96]通过长期浸出试验研究了温度和淋溶体积与Cd浓度的关系,表明在夏季温度和降雨量高于其他季节时,Cd具有较高的浸出率。此外,模拟酸雨(pH=4.5)条件下有利于颗粒表面部分可交换态结合的Cd浸出[97-98]。因此,气化细渣在填埋处理过程中Cd会对环境造成风险。

图8 不同pH下Cd的浸出率[92]
Fig.8 Leaching ratio of Cd under different pH[92]
2.4 铅(Pb)
不同pH下Pd浸出率如图9所示,图9中,>115、>75~115、>38~75、 ≤38 μm的气化渣分别被命名为SGPS-A~SGFS-D。Pb在低pH和高pH下均表现出高浸出率,这种特点被定义为两性浸出行为[91]。QUINA等[28]观察到Pb的浸出率首先随萃取液pH的升高而降低,在接近中性范围内浸出率最小,最后浸出率随pH升高而升高。作为一种两性金属,Pb的最低值溶解度发生在pH=9左右,此后由于溶液中形成更多可溶性阴离子羟基络合物使Pb溶解度增加,并在pH>11.5时显著增加[99]。受灰渣种类影响,CORNELIS等[100]发现Pb最小浸出率对应pH存在差异。此外,气化渣中Pb浸出动力学表明浸出活化能总是随粒度分数的降低而降低,降低粒径有利于提高Pb浸出率[18]。当渗滤液pH低于4或大于12时,气化灰渣浸滤液中的Pb浓度超过了GB/T 14848—2017《地下水质量标准》中III级地下水质量限值[30]。因此,作为一种两性金属,在处置或利用气化灰渣时,应关注Pb在酸性条件和碱性条件下的潜在环境危害。

图9 不同pH值下Pb的浸出率[18]
Fig.9 Leaching ratio ofPb under different pH [18]
2.5 锌(Zn)
金属阳离子Zn 更易溶于较低 pH 酸浸出,其浸出率随着pH降低而逐渐增加,具体如图10所示。Zn在煤气化过程中挥发至煤气中,并随煤气冷却附着在细渣表面上,因而更易被酸浸出[19,101]。浸出模型表明Zn的浸出过程由扩散模型控制,并根据浸出活化能可以得出,Zn的浸出需要克服更少的能量障碍;灰渣中高酸溶性和可交换部分的Zn,使其更易在低pH溶液中浸出;Zn的浸出活化能随粒径的减小而降低,减小灰渣粒径有利于Zn浸出[18]。模拟酸雨(pH=4.5)条件下有利于颗粒表面可交换部分结合的Zn浸出[97-98]。此外,Fe3 相的溶解可能会导致Zn同时浸出,增加Zn浸出率[102]。因此,气化灰渣在填埋处理过程应注意Zn对环境造成风险。

图10 不同pH下Zn的浸出率[18]
Fig.10 Leaching ratio of Zn under different pH[18]
2.6 镍(Ni)
Ni对浸出液pH值较为敏感,其浸出模式属于阳离子模式[103],如图11所示。NIU等[92]发现,煤气化渣中Ni在酸性条件下由不溶转化为微溶,其浸出率随浸出液初始酸度的增加而增加,如图8所示。DU等[29]对酸性浸出条件下Ni的浸出行为进行系统研究,发现醋酸(HAC)和盐酸(HCl)对Ni的浸出有显著影响,并且在7 mol/L HAC和3 mol/L HCl浸出液条件下可以去除灰渣中Ni。煤气化渣中,细渣中Ni的浸出能力高于粗渣,这是由于小尺寸的灰渣颗粒中具有多孔隙结构和高比表面积,提高了Ni的浸出效率[73,104]。Ni的浸出行为受扩散模型控制,升高温度有利于降低浸出活化能,减小粒径有利于降低浸出活化能[18]。此外,灰渣的酸碱比((SiO2 Al2O3)/(CaO Fe2O3))也可以作为衡量Ni浸出能力的重要指标,随酸碱比升高有利于Ni浸出,并在酸碱比为2时达到最大浸出率[29]。因此,在处理高Ni气化渣时应关注pH和酸碱比对Ni的浸出影响。

图11 不同pH下Ni的浸出率[18]
Fig.11 Leaching ratio of Ni under different pH[18]
3 气化灰渣中重金属的固化脱毒
煤气化渣中重金属(除Hg外)浓度明显高于进料煤中重金属浓度,自然降水会导致未经处理的气化渣中重金属溶解浸出,污染当地生态环境[19]。因此,对气化渣进行填埋堆积、综合利用过程中,应采用各种方法将重金属固定在灰渣中,降低重金属浸出量,避免对环境造成不利影响。目前,重金属固化技术已经成为主流重金属脱毒方法之一,并在固废处理中得到广泛应用[105]。气化灰渣中重金属通过一系列物理化学变化被固化在矿物中,降低重金属的浸出率,减少对环境的污染,对实现灰渣的无害化利用有重要意义。
3.1 水泥固化
煤气化渣在水泥建材行业中的应用是资源化利用的重要途径之一。煤气化灰渣的主要化学成分为SiO2、Al2O3和CaO等,从化学组成看,可作为生产水泥熟料的原料,并已广泛用于水泥生产[3]。刘开平等[33]使用气化粗渣作为混凝土中的细骨料制备混凝土,其抗压强度远高于基准混凝土,并且强度随着时间推移不断提高。将一定比例气化灰渣制备发泡水泥,具有质轻、保温、防火、抗冻性好等特点,可满足墙体保温材料的性能要求[106]。水泥可以稳定气化灰渣中的重金属污染物,起到重金属脱毒作用。重金属在水泥固化过程中主要包括2种模式,一种是重金属在固化过程中被C-S-H凝胶包裹;另一种是重金属进入Si—Al网络结构并形成M—Al化学键以平衡电荷[107]。BIE等[108]研究了水泥用量、浸出液pH和振动浸出时间对重金属淋溶量的影响,发现随水泥用量增加、pH和振动时间减少,气化灰渣中重金属的浸出速率降低。MAO等[109]用脱硫石膏和铝灰在1 270 ℃下制备水泥,Zn、Cd、Pb等重金属离子的浸出浓度远低于国家标准限值。
然而,气化灰渣用于水泥行业还存在难点。如气化灰渣合成水泥的水化产物在受酸雨影响的地区会溶解,导致其钝化能力降低,水泥中重金属存在浸出风险[110]。干湿循环、冻融循环和其他气候条件可能会影响气化灰渣合成水泥中重金属稳定效果[111]。此外,煤气化渣含有较高残碳,阻碍灰渣与水泥发生胶凝反应,影响二者结合和水泥性能。因此,水泥可以固化重金属起到脱毒作用,但对煤气化渣中残碳含量有较高要求,优化煤气化渣残碳分选工艺将是未来发展方向[13,112]。
3.2 热处理固化
热处理固定气化渣中重金属原理是通过高温反应促进化学键合、元素置换和晶相中新组分的形成,达到脱毒的目的[113]。气化细渣的小粒径和还原性,决定了气化细渣可以作为一种有效的添加剂,不仅在玻璃、陶瓷烧制过程中改善烧结过程,而且烧制出的陶瓷试件具有较好烧结性能,吸水率、饱和系数、机械强度均比未加气化细渣的标准件有所提高[114]。MA等[115]通过热力学计算确定了低熔点的混合比例,并进行烧结试验,结果表明Al2O3有助于Cr、Cu和Ni的固定降低重金属综合毒性指数。张凯等[116]利用煤气化粗渣,改进免烧方式并制备出陶粒,符合环境安全标准;经浸出毒性测试后,发现免烧工艺能够对煤气化粗渣中重金属起到固定作用,有良好的市场化前景。此外,熔渣中熔融相含量与玻璃相含量呈正相关,熔化温度升高不利于重金属的凝固。
值得注意的是,高温热处理将消耗大量能量,采取相应措施(如低熔点矿物配方、添加助熔剂等)降低气化灰渣熔点,减少能源消耗,将使气化灰渣玻璃陶瓷化处理过程更加节能环保。
3.3 水热处理固化
水热处理固化是指在水热条件下将固体废物在某一特定条件下进行离子交换或与添加剂反应,将重金属固定在矿物中,并生成可利用的材料(分子筛、催化剂、吸附剂等)[117],被认为是最有前途的固废回收方法之一,也是制备建筑材料的新技术[118-119]。水热处理作为煤气化渣的脱毒方法,可以固定和重新分配重金属(Zn,Pb,Cr,Cd)和其他有害元素[120]。在最佳试验条件下(260 ℃,4 h),Zn的硫化程度大于92%,硫化物的生成将有利于重金属的稳定和回收[121-123]。同时,水热法与添加剂结合使用有利于促进重金属向更稳定化转变。相对于常规水热处理,将硅铝添加剂与灰渣混合水热处理,可以有效降低Cd、Zn、Cr和Pb等重金属浸出率[35]。胡艳军等[124]利用水热法耦合碳酸钠技术实现重金属的稳定固化,显著降低了水热后灰渣中重金属的迁移性。不仅如此,水热法还可作为一种新技术将灰渣转化为可利用材料[125-126]。对石灰含量较高的水冷渣体,在水热反应中通过添加石英或粉煤灰,能够直接生成硅酸盐水泥等建筑材料,实现废渣利用和重金属固化脱毒一体[127]。此外,灰渣水热反应后的吸附能力也将显著增强,可作为重金属吸附剂使用。硅铝酸盐灰渣混合物在220 ℃下水热处理6 h,其产物具有吸附废水中重金属阳离子的潜力,可以从工业废水中吸附Cr3 ,吸附效率为98%[128]。
值得注意的是,水热处理后的浸出液如何处理、使用易被忽视。浸出液通常含有重金属,因此需对水热处理过程中产生的有毒有害溶液进行处理。
3.4 机械混合固化
添加剂固化是指采用机械手段将固体废物与添加剂(硅铝化合物、废渣等)在一定条件下混合,实现灰渣中重金属的固化。在外加高强度和长时间的机械力作用下,灰渣和添加剂会发生团聚现象,混合产物的颗粒尺寸变大、孔隙直径变大,使吸附在表面的重金属被包裹其中,增强了重金属的包裹稳定化效果,减少了重金属浸出量[129]。LI等[130]在灰渣中添加硅粉,经过球磨、水化等作用,获得水化硅酸钙(C-S-H)产物,有效降低了灰渣中重金属的浸出。黎雯等[131]将膨润土、高岭土、硅灰、赤泥等添加剂与焚烧灰渣机械混合,提高飞灰中Pb、Zn的稳定态含量,提高混合灰中Cr残渣态的含量,有效降低灰渣中重金属浸出率。另一方面,在机械力和一定条件(水化、水热)共同作用下,灰渣和添加剂的表面结构破碎,化学键断裂重组,在此过程中产生的原子基团或形成的不饱和悬空键会促进混合物的表面活性增强,使其在该条件下形成新的晶体结构(如水铝钙石、硅钙石、硅酸盐等),此类物质可将重金属包裹其中,转变成更稳定的形态[131]。
除硅铝添加剂外,工业废渣(碱渣、钢渣)与灰渣混合也能够固化灰渣中重金属,降低重金属的浸出率,此技术已在土壤中重金属固定和修复得到运用[132]。ZHA等[133]发现碱渣具有吸附Pb作用,与灰渣混合可以固定灰渣中的Pb,减少Pb析出。在酸性环境中,碱渣有助于灰渣中矿物的水合作用,保持灰渣中重金属的稳定。YANG等[134]研究发现钢渣可用于混合其他高重金属固体废物,具有凝固或稳定重金属(Cd、Pb、Zn、Cu等)的作用。对于Pb和Cd污染的区域,使用钢渣会降低环境中Pb和Cd离子的交换能力[135]。此外,钢渣还可通过沉淀和吸附作用将环境中的Cd离子转化为不易分解的CdCO3、CdO和Cd(OH)2[136]。尽管使用工业废渣处理灰渣具有很大潜力,但这些工业废渣中有害元素带来的生态和环境风险不容忽视,如Hg、Cd、As、Pb、Cr、Ni、Cu和Zn也存在于碱渣[137]。
3.5 化学沉淀固化
化学沉淀法是指在固体废渣中加入化学试剂,形成废渣中重金属元素脱出、回收、利用的完整工艺。化学沉淀不仅可以减少灰渣中重金属对环境危害,更注重灰渣中重金属回收利用,提高某些金属(如Cr)的高附加值[107]。对于Cr含量较高的固体废物,化学沉淀法可以用来脱出和利用浸出液中的Cr3 [138]。Cr3 在酸浸出液中与Fe2 和Al3 的水解pH相似,仅通过水解沉淀很难将其分离出来[93]。ISHFAQ等[139]通过先添加氧化剂将浸出液中的Fe2 提前氧化成Fe3 ,再降低浸出液的水解pH,可以实现Cr3 逐步分离提取,并且随pH升高,Cr3 的脱出率逐渐增加。QU等[94]系统研究浸出液pH对Cr3 脱出影响,发现pH在1.6~2.0时,Cr3 脱出率在70.58%~71.69%;pH为4.0时,Cr3 的去除率为98.34%。然而,pH进一步升高时,Al3 会发生水解,使Cr3 和Al3 难以分离。基于上述研究结果,提出了煤气化渣酸浸液的脱毒机理:首先进行选择性氧化,将Fe2 氧化成Fe3 ,在这个过程中,Cr3 不被氧化;然后通过调节pH进行共沉淀,形成Fe-Cr共沉淀并从酸浸溶液中分离Al3 ;最后共沉淀后,过滤得到脱毒酸浸液,残留物为Fe-Cr共沉淀物,将被进一步回收利用[94]。其反应过程如下[94]:
2Fe2 2H H2O2 ![]()
2Fe3 2H2O(选择性氧化),
(1)
Fe3 3OH- ![]() Fe(OH)3(共沉淀),
Fe(OH)3(共沉淀),
(2)
Cr3 3OH-![]() Cr(OH)3(共沉淀),
Cr(OH)3(共沉淀),
(3)
mFe(OH)3 nCr(OH)3![]()
(m n)Fem/(m n)Crn/(m n)OOH (m n)H2O(共沉淀物)。
(4)
3.6 矿化协同固化
灰渣矿化协同固化重金属处理技术不仅能够通过固定CO2来减少温室气体排放,还能将重金属固定在矿物中降低重金属的浸出率,现已成为灰渣处理的热门技术之一[140-141]。煤气化渣的化学成分与粉煤灰类似,主要由SiO2、Al2O3、Fe2O3、CaO和MgO以及一些重金属构成,其中部分CaO可以与CO2反应并将其固定在灰渣中[142]。同时,灰渣碳酸化会使灰渣pH>10下降至pH=8~9,为大多数重金属,特别是两性重金属(Pb)浸出率最低的pH区间;并且,灰渣中金属阳离子(如Pb2 、Zn2 、Cd2 )会与![]() 反应生成碳酸盐沉淀,降低重金属的浸出率[143-144]。杨刚[145]研究表明灰渣矿化后Pb的浸出率减少48.9%,Cr的浸出率减少96.4%,Cd的浸出率减少87.0%。重金属的固化率受矿化时间、温度、压力和固液比等因素影响。增加矿化时间和压力有利于重金属固化,但过长时间和过高压力对重金属固化作用不大;在一定温度范围内,提高温度有利于重金属矿化,超过此范围提高温度会使CO2溶解度减少,金属自由离子运动加剧,不利于重金属固化;固液比对重金属的固化作用类似温度,存在一个最佳值,该值受灰渣理化性质影响[140,143,145]。此外,矿化协同固化重金属时应注意酸性气体(如SO2)的进入,其会影响矿化协同作用,进而降低重金属的固化率[146]。
反应生成碳酸盐沉淀,降低重金属的浸出率[143-144]。杨刚[145]研究表明灰渣矿化后Pb的浸出率减少48.9%,Cr的浸出率减少96.4%,Cd的浸出率减少87.0%。重金属的固化率受矿化时间、温度、压力和固液比等因素影响。增加矿化时间和压力有利于重金属固化,但过长时间和过高压力对重金属固化作用不大;在一定温度范围内,提高温度有利于重金属矿化,超过此范围提高温度会使CO2溶解度减少,金属自由离子运动加剧,不利于重金属固化;固液比对重金属的固化作用类似温度,存在一个最佳值,该值受灰渣理化性质影响[140,143,145]。此外,矿化协同固化重金属时应注意酸性气体(如SO2)的进入,其会影响矿化协同作用,进而降低重金属的固化率[146]。
4 结语和展望
针对煤中6种典型有毒重金属(Hg、Cr、Cd、Pb、Zn、Ni),概述了其在煤气化过程中迁移行为和影响因素。重金属的迁移随挥发难易程度呈现明显差异,其中难挥发元素Cr主要富集于粗渣中,半挥发元素Cd和Ni在粗渣和细渣中均有富集,挥发元素Hg、Pb和Zn则富集于细渣中。气化温度是影响重金属迁移释放的主要因素。升高温度有利于重金属的释放,增大气化压力则降低重金属释放率;进一步分析了重金属在灰渣中的赋存形态和分布特点,总结了气化灰渣浸出过程中的重金属浸出行为及其影响因素,其中浸出液pH是主要因素,Cr、Cd、Ni和Zn在酸性条件下具有较高的浸出率,而Pb具有两性浸出行为,在酸性和碱性条件下浸出率均较高,在中性条件附近浸出率达到最低;最后分类梳理了现有煤气化渣中重金属固化方法原理和进展,概述水泥固化、热处理固化、水热法固化、添加剂固化、化学共沉淀固化和矿化协同固化等6种固化方法的优势和不足。然而,以往研究在重金属的释放比较、长期浸出行为、固化回收综合利用方面研究较少,需在今后进一步深入研究:
1)在煤气化过程中重金属释放迁移方面,应进一步完善不同气化技术对重金属释放影响。原料煤的输入形式和气化反应条件直接影响煤中重金属的释放和迁移。煤气化技术分为固定床气化技术、流化床气化技术、气流床气化技术、地下煤气化技术和等离子体煤气化技术等[6,147]。然而,不同煤气化技术对重金属释放行为研究深度各异,缺少系统的理论研究,需进一步完善不同煤气化工艺重金属迁移转化理论和试验研究。
2)在煤气化渣中重金属浸出行为方面,应进一步完善长期环境对重金属浸出行为影响。以往对气化渣中重金属的浸出行为研究集中在短期浸出研究,通过直接改变浸出液条件(pH、温度、固液比等),得到重金属浸出行为,对重金属的长期浸出行为研究较少。特别是,煤气化渣在填埋、堆积、作为路基和建材等过程中,受到长期环境影响(降雨量、雨水pH、季节温度等),其浸出行为可能发生变化。
3)在煤气化渣中重金属固化方面,应进一步优化固化方法和探索分级固化、多种固化相结合利用方式。优化分选工艺,降低灰渣中残碳含量,达到利用标准;优化玻璃、陶瓷制备工艺,通过配比低熔点矿物组分来降低熔点,进而降低能耗;优化添加固化剂机械固化,与其他固体废物相结合,如钢渣、煤矸石等,达到固体的废物系统处理目的;探索水热处理和化学沉淀一体化固化提纯方法,将难溶重金属固定在灰渣中,而易溶重金属通过化学沉淀从废液中分离,达到固定和提取结合的效果。
4)在煤气化渣中重金属处理方面,探索煤气化渣中重金属的资源化利用,建立控制—回收—提纯—利用一体化处理。以往研究主要针对重金属的固化处理,对重金属回收利用研究较少,无法提升灰渣中重金属的额外价值。如高Cr含量粗渣为Cr的回收利用提供大量廉价材料,回收提纯Cr不仅减少环境污染,还可制备出高附加值产品。
[1] 国家统计局. 2022年我国能源生产和消费相关数据[EB/OL].[2023-06-04]. https://www.stats.gov.cn/.
[2] 王冀, 孔令学, 白进, 等. 煤气化灰渣中残炭对灰渣流动性影响的研究进展[J]. 洁净煤技术, 2021, 27(1):181-192.
WANG Ji,KONG Lingxue,BAI Jin. et al. Research progress on the effbct of residual carbon in coal gasification slag on ash and slag now property [J]. Clean Coal Technology. 2021, 27(1):181-192.
[3] 曲江山, 张建波, 孙志刚, 等. 煤气化渣综合利用研究进展[J]. 洁净煤技术, 2020, 26(1):184-193.
QU Jiangshan, ZHANG Jianbo, SUN Zhigang, et al. Reserch progress on comprehensive utilization of coal gasification slag [J]. Clean Coal Technology, 2020, 26(1): 184-193.
[4] LIU X D, JIN Z W, JING Y H, et al. Review of the characteris-tics and graded utilisation of coal gasification slag [J]. Chinese Journal of Chemical Engineering, 2021, 35:92-106.
[5] 史达, 张建波, 杨晨年, 等. 煤气化灰渣脱碳技术研究进展[J]. 洁净煤技术, 2021, 41(4):1-10.
SHI Da, ZHANG Jianbo, YANG Chennian, et al. Research progress of the decarburization technology of coal gasification ash slag [J]. Clean Coal Technology, 2021, 41(4):1-10.
[6] 王辅臣. 煤气化技术在中国:回顾与展望 [J].洁净煤技术, 2021, 27(1):1-33.
WANG Fuchen. Coal gasification technologies in China: Review and prospect [J]. Clean Coal Technology, 2021, 27(1):1-33.
[7] 王利峰. 我国煤气化技术发展与展望[J]. 洁净煤技术, 2022, 28(2):115-121.
WANG Lifeng. Development and prospect of coal gasification technology in China[J]. Clean Coal Technology, 2022,28(2):115-121.
[8] GUO Y, GUO F, ZHOU L, et al. Investigation on co-combustion of coal gasification fine slag residual carbon and sawdust char blends: Physiochemical properties, combustion characteristic and kinetic behavior [J]. Fuel, 2021, 292: 120387.
[9] 于伟, 王学斌, 白永辉, 等. 煤气化细渣浮选脱碳试验研究[J]. 洁净煤技术, 2021, 27(3):81-87.
YU Wei, WANG Xuebin, BAI Yonghui, et al. Experimental study on decarboIlization of coal gasification fine slag by notation [J]. Clean Coal Technology, 2021, 27(3):81-87.
[10] 周安宁, 高影, 李振, 等. 煤气化灰渣组成结构及分选加工研究进展[J]. 西安科技大学学报, 2021, (4):575-584.
ZHOU Anning,GAO Ying,LI Zhen, et al. Composition structure and separation processing of ash and slag during coal gasification [J]. Journal of Xi′an University of Science and Technology, 2021, (4):575-584.
[11] 王文钰, 李伟, 梁晨, 等. 西北地区气流床煤气化细灰理化特性研究[J]. 洁净煤技术, 2021, 27(3):94-100.
WANG Wenyu, LI WEi, LIANG Chen, et al. Research on physicochemical characteristics of fine slag ftom gasification in Northwest China [J]. Clean Coal Technology, 2021, 27(3):94-100.
[12] REN L, DING L, GUO Q, et al. Characterization, carbon-ash separation and resource utilization of coal gasification fine slag:A comprehensive review [J]. Journal of Cleaner Production, 2023, 398:136554.
[13] 宁永安, 段一航, 高宁博, 等. 煤气化渣组分回收与利用技术研究进展[J]. 洁净煤技术, 2020, 26(S1):14-19.
NING Yong′an, DUAN Yihang, GAO Ningbo, et al.Progress of component recycling and utilization technology of coal gasification slag [J]. Clean Coal Technology, 2020, 26(S1):14-19.
[14] 范宁, 张逸群, 樊盼盼, 等. 煤气化渣特性分析及资源化利用研究进展[J]. 洁净煤技术, 2022, 28(8):145-154.
FAN Ning, ZHANG Yiqun, FAN Panpan, et al. Research progress on characteristic analysis and resource utilization of coal gasification slag [J]. Clean Coal Technology, 2022, 28(8):145-154.
[15] FU B, CHENG Z, WANG D, et al. Investigation on the utilization of coal gasification slag in Portland cement:Reaction kinetics and microstructure [J]. Construction and Building Materials, 2022, 323:126587.
[16] MIAO Z, WU J, QIU G, et al. Solving two industrial waste iss-ues simultaneously:Coal gasification fine slag-based hierarchical porous composite with enhanced CO2 adsorption performance [J]. Science of the Total Environment, 2022, 821:153347.
[17] 侯志勇, 郝亮亮, 沈小瑞, 等. 煤气化细渣制备吸附材料研究进展[J]. 洁净煤技术, 2021, 27(S2):201-205.
HOU Zhiyong, HAO Liangliang, SHEN Xiaorui, et al. Research progress on preparation of adsorption materials from coal gasification fine slag [J]. Clean Coal Technology, 2021,27(S2):201-205.
[18] GUO Y, ZHANG Y, ZHAO X, et al. Multifaceted evaluation of distribution, occurrence, and leaching features of typical heavy metals in different-sized coal gasification fine slag from Ningdong region, China:A case study [J]. Science of the Total Environment, 2022, 831:154726.
[19] JIANG P, XIE C R, LUO C L, et al. Distribution and modes of occurrence of heavy metals in opposed multi-burner coal-water-slurry gasification plants [J]. Fuel, 2021, 303:121163.
[20] GUO Y, MA C, ZHANG Y, et al. Comparative study on the structure characteristics, combustion reactivity, and potential environmental impacts of coal gasification fine slag with different particle size fractions [J]. Fuel, 2022, 311:122493.
[21] 黄亚继. 煤气化过程中痕量元素迁移规律与气化温度的关系[J]. 中国电机工程学报, 2006, 26(4):10-15.
HUANG Yaji. The relationship between occurrence of trace elements and gasification temperature [J]. Proceedings of the CSEE, 2006, 26(4):10-15.
[22] 黄亚继, 金保升, 仲兆平, 等. 空煤比对煤气化过程中痕量元素迁移规律的影响[J]. 热能动力工程, 2004(4):402-407.
HUANG Yaji, JIN Baosheng, ZHONG Zhaoping, et al. Effect of empty coal ratio on the migration pattern of trace elements in coal gasification process [J]. Journal of Engineering for Thermal Energy and Power, 2004(4):402-407.
[23] 黄亚继, 金保升, 仲兆平, 等. 气化压力对煤气化过程中痕量元素迁移规律的影响[J]. 东南大学学报(自然科学版), 2008, 38(1):92-96.
HUANG Yaji, JIN Baosheng, ZHONG Zhaoping, et al. Effect of gasification pressure on the occurrence of trace elements [J]. Journal of Southeast University(Natural Science Edition), 2008,38(1):92-96.
[24] MIAO Z K, WU J J, ZHANG Y X, et al. Physicochemical characteristics of mineral-rich particles present in fine slag from entrained-flow gasifiers [J]. Energy &Fuels, 2020, 34(1):616-623.
[25] GUO F H, MIAO Z K, GUO Z K, et al. Properties of flotation residual carbon from gasification fine slag [J]. Fuel, 2020, 267:117043.
[26] PAN C C, LIANG Q F, GUO X L, et al. Characteristics of different sized slag particles from entrained-flow coal gasification [J]. Energy &Fuels, 2016, 30(2):1487-1495.
[27] DU M J, LIU H, HU D H, et al. The leaching mechanism of heavy metals (Ni, Cd, As) in a gasification slag during acidification [J]. Waste Management, 2020, 114:17-24.
[28] QUINA M J, BORDADO J C M, QUINTA-FERREIRA R M. The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues [J]. Waste Management, 2009, 29(9):2483-2493.
[29] DU M, LIU H, HU D, et al. The leaching mechanism of heavy metals (Ni, Cd, As) in a gasification slag during acidification [J]. Waste Management, 2020, 114:17-24.
[30] GUO Y, ZHANG Y X, ZHAO X, et al. Multifaceted evaluation of distribution, occurrence, and leaching features of typical heavy metals in different-sized coal gasification fine slag from Ningdong region, China:A case study [J]. Science of The Total Environment, 2022, 831:154726.
[31] ZHAO S L, DUAN Y F, LU J C, et al. Thermal stability, chemical speciation and leaching characteristics of hazardous trace elements in FGD gypsum from coal-fired power plants [J]. Fuel, 2018, 231:94-100.
[32] ZANG F, WANG S L, NAN Z R, et al. Leachability of heavy metals in loess-amended dredged sediment from Northwest of China [J]. Ecotoxicology and Environmental Safety, 2019, 183:109561.
[33] 刘开平, 赵红艳, 李祖仲, 等. 煤气化渣对水泥混凝土性能的影响[J]. 建筑科学与工程学报, 2017, 34(5):190-195.
LIU Kaiping, ZHAO Hongyan, LI Zuzhong, et al. Influence ofcoal gasification slag on cement concrete performance [J]. Journal of Architecture and Civil Engineering, 2017, 34(5):190-195.
[34] 赵永彬, 吴辉, 蔡晓亮, 等. 煤气化残渣的基本特性研究[J]. 洁净煤技术, 2015, 21(3):110-113.
ZHAO Yongbin, WU Hui, CAI Xiaoliang, et al. Basic characteristics of coal gasification residual [J]. Clean Coal Technology, 2015, 21(3):110-113.
[35] SHI D, HU C, ZHANG J, et al. Silicon-aluminum additives assisted hydrothermal process for stabilization of heavy metals in fly ash from MSW incineration [J]. Fuel Processing Technology, 2017, 165:44-53.
[36] CLARKE L B. The fate of trace elements during coal combustion and gasification:An overview [J]. Fuel, 1993, 72(6):731-736.
[37] STREETS D G, HOROWITZ H M, JACOB D J, et al. Total me-rcury released to the environment by human activities [J]. Environmental Science &Technology, 2017, (51):5969-5977.
[38] LIU S Q, WANG C H, LIU M Y, et al. Distribution and fate of trace elements during fixed-bed pressurized gasification of lignite [J]. Energy &Fuels, 2016, 30(11):9061-9070.
[39] GALBREATH K C, ZYGARLICKE C J. Mercury transformations in coal combustion flue gas [J]. Fuel Processing Technology, 2000, 65:289-310.
[40] 余琛. 煤中汞、氯、氟释放和形态转化规律的研究[D]. 武汉:华中科技大学, 2009.
[41] WU C Y, BISWAS P. An equilibrium analysis to determine the speciation of metals in an incinerator [J]. Combustion and Flame, 1993, 93(1/2):31-40.
[42] MOJTAHEDI W, LYYRäNEN J, JOKINIEMI J, et al. Trace element partitioning during coal gasification [J]. Fuel, 1996, 75:931-939.
[43] BUNT J R, WAANDERS F B. Trace element behaviour in the Sa-sol-Lurgi MK IV FBDB gasifier. Part 1:The volatile elements:Hg, As, Se, Cd and Pb [J]. Fuel Guildford, 2008, 87:2374-2387.
[44] WAN Z X, DUAN L Y, HU X D, et al. Removal of mercury from flue gas using coal gasification slag [J]. Fuel Processing Technology, 2022, 231:107258.
[45] 王云鹤, 李海滨, 黄海涛, 等. 重金属在煤气化过程的分布迁移规律及控制[J]. 中国环境科学, 2002, 22(6):556-560.
WANG Yunhe, LI Haibin, HUANG Haitao, et al. Distribution rule and control of heavy metals during coal gasification [J]. China Environmental Science, 2002, 22(6):556-560.
[46] NANDY A, LOHA C, GU S, et al. Present status and overview of Chemical Looping Combustion technology [J]. Renewable and Sustainable Energy Reviews, 2016, 59:597-619.
[47] NIU P J, MA Y X, TIAN X, et al. Chemical looping gasification of biomass:Part I. Screening Cu-Fe metal oxides as oxygen carrier and optimizing experimental conditions [J]. Biomass and Bioenergy, 2018, 108:146-156.
[48] JAIN A, SEYED-REIHANI S A, FISCHER C C, et al. Ab initio screening of metal sorbents for elemental mercury capture in syngas streams[J]. Chemical Engineering Science, 2010, 65:3025-3033.
[49] AN M, MA J J, GUO Q J. Transformation and migration of mercury during chemical-looping gasification of coal [J]. Industrial & Engineering Chemistry Process Design and Development, 2019, 58(44):20481-20490.
[50] BUNT J R, WAANDERS F B. Trace element behaviour in the Sasol-Lurgi fixed-bed dry-bottom gasifier. Part 3:The non-volatile elements:Ba, Co, Cr, Mn, and V [J]. Fuel, 2010, 89(3):537-548.
[51] FRANDSEN F, DAM-JOHANSEN K, RASMUSSEN P. Trace elements from combustion and gasification of coal:An equilibrium approach [J]. Progress in Energy &Combustion Science, 1994, 20(2):115-138.
[52] JIANG P, XIE C R, LUO C L, et al. Distribution and modes of occurrence of heavy metals in opposed multi-burner coal-water-slurry gasification plants [J]. Fuel, 2021, 303(3):121163.
[53] 李玉兰, 刘鑫, 邵素军, 等. 煤炭地下气化灰渣浸泡实验及Zn、Cd、Pb、As环境效应分析[J]. 安全与环境学报, 2008, 8(2):80-82.
LI Yulan, LIU Xin, SHAO Sujun, et al. Leaching test of Zn,Cd,Pb,As in UCG residues for the environmental impact assessment [J]. Journal of Safety and Environment, 2008, 8(2):80-82.
[54] YUAN L, LIU G, QI C, et al. Chemical speciation and combustion behavior of chromium (Cr) and vanadium (V) in coals [J]. Fuel, 2016, 184:42-49.
[55]  L, RACLAVSK
L, RACLAVSK H, ECH B, et al. Behavior of Cd during coal combustion:An overview [J]. Processes, 2020, 8:1237.
H, ECH B, et al. Behavior of Cd during coal combustion:An overview [J]. Processes, 2020, 8:1237.
[56] BARTONOVA L, RACLAVSKA H. Behavior of Cr during coal combustion:An overview [J]. Fuel, 2022, 322:124210.
[57] LANE D J, SIPPULA O, PERANIEMI S, et al. Detoxification of wood-combustion ashes containing Cr and Cd by thermal treatment [J]. Journal of Hazardous Materials, 2020, 400:123315.
[58] 吕海亮, 陈皓侃, 李文, 等. 义马煤中几种微量污染元素的赋存形态研究[J]. 燃 料化学学报, 2003, 31(1):31-34.
LYU Hailing, CHEN Haokan, LI Wen, et al. Mode of occurrence of several trace elements in Yima coal [J]. Journal of Fuel Chemistry and Technology, 2003,31(1):31-34.
[59] GUO R X, YANG J L, LIU D Y, et al. Transformation behavior of trace elements during coal pyrolysis [J]. Fuel Process Technology, 2002, 77(25):137-143.
[60] CHEN G Y, SUN Y N, YAN B B, et al. Distribution of trace elements during coal gasification :The effect of upgrading method [J]. Journal of Cleaner Production, 2018, 190:193-199.
[61] 周玲妹, 郭豪, 初茉, 等. 低阶煤中镉的赋存对其在热解中释放的影响[J]. 煤炭学报, 2019, 44(1):323-331.
ZHOU Lingmei, GUO Hao, CHU Mo, et al. Eeffects of occurrence mode of Cd in low rank coal on its volability during pyrolysis process [J]. Journal of China Coal Society, 2019,44(1):323-331.
[62] WANG Y F, TANG Y G, LI R Q, et al. Measurements of the leachability of potentially hazardous trace elements from solid coal gasification wastes in China [J]. Science of the Total Environment, 2021, 759:143463.
[63] FINKELMAN R B, PALMER C A, WANG P P. Quantification of the modes of occurrence of 42 elements in coal [J]. International Journal of Coal Geology, 2018, 185:138-160.
[64] SCHELLENBERG W, ULLRICH N. Prenflo-pressurised coal-gasification [J]. Sixth Annual International Pittsburgh Coal Conference, 1989, 128:507-516.
[65] WANG L, ZHENG C H, ZHANG Y X, et al. Speciation characteristics and mobility of trace elements across ultralow emission air pollution control devices[J]. Energy &Fuels, 2017, 31(12):13963-13971.
[66] WILLIAM P Linak, JOST OL Wendt. Trace metal transformation mechanisms during coal combustion [J]. Fuel Processing Technology, 1994, 39:173-198.
[67] THOMPSON D, ARGENT B B. Prediction of the distribution of trace elements between the product streams of the Prenflo gasifier and comparison with reported data [J]. Fuel, 2002, 81(5):555-570.
[68] GUO R X, YANG J L, LIU D Y, et al. Transformation behavior of trace elements during coal pyrolysis [J]. Fuel Processing Technology, 2002, 77:137-143.
[69] WANG K C, ZHANG J, SHANG C, et al. Operation optimi-zation of shell coal gasification process based on convolutional neural network models [J]. Applied Energy, 2021, 292:116847.
[70] MOJTAHEDI W, LARJAVA K. Fate of some trace elements in combustion and gasification processes[J]. Environmental Technology, 1987:323-333.
[71] CHEN Y, CHEN F, ZHOU F, et al. Early solidification/stabilization mechanism of heavy metals (Pb, Cr and Zn) in shell coal gasification fly ash based geopolymer [J]. Science of the Total Environment, 2022, 802:149905.
[72] BUNT J R, WAANDERS F B. Trace element behaviour in the Sasol-Lurgi MK IV FBDB gasifier. Part 2:The semi-volatile elements:Cu, Mo, Ni and Zn [J]. Fuel, 2009, 88(6):961-969.
[73] JIANG P, XIE C R, LUO C L, et al. Distribution and modes of occurrence of heavy metals in opposed multi-burner coal-water-slurry gasification plants [J]. Fuel, 2021, 303:121163.
[74] ZHANG S R, JIANG X G, LIU B X, et al. Co-combustion of bituminous coal and pickling sludge in a drop tube furnace:Thermodynamic study and experimental data on the distribution of Cr, Ni, Mn, As, Cu, Sb, Pb, Cd, Zn, and Sn [J]. Energy &Fuels, 2017, 31(3):3019-3028.
[75] ZHOU Y M, YANG J P, DONG L C, et al. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres from fly ash:Part 5. Industrial scale studies at a 50 MWth coal-fired power plant[J]. Fuel, 2020, 266:117052.
[76] ZHANG Y, YANG J P, YU X H, et al. Migration and emission characteristics of Hg in coal-fired power plant of China with ultra low emission air pollution control devices[J]. Fuel Processing Technology, 2017, 158:272-280.
[77] MA W, LI Z, LV J, et al. Environmental evaluation study of toxic elements (F, Zn, Be, Ni, Ba, U) in the underground coal gasification (UCG) residuals [J]. Journal of Cleaner Production, 2021, 297:126565.
[78] NIU M, FU Y, LIU S. Distribution and leachability of hazardous trace elements in Lurgi gasification ash from a coal - to - SNG plant [J]. Journal of the Energy Institute, 2021, 98:223-233.
[79] TANG Y, GUO X, XIE Q, et al. Petrological characteristics and trace element partitioning of gasification residues from slagging entrained-flow gasifiers in Ningdong, China [J]. Energy &Fuels, 2018, 32(3):3052-3067.
[80] FRANDSEN F, DAM-JOHANSEN K, RASMUSSEN P. Trace elements from combustion and gasification of coal:An equilibrium approach [J]. Progress in Energy and Combustion Science, 1994, 20(2):115-138.
[81] SENIOR C L, III L E B, MORENCY J R. Laboratory study of trace element vaporization from combustion of pulverized coal [J]. Fuel Processing Technology, 2000, 63(2/3):109-124.
[82] KOSSON D S, GARRABRANTS A C, DELAPP R, et al. pH-dependent leaching of constituents of potential concern from concrete materials containing coal combustion fly ash [J]. Chemosphere, 2014, 103:140-147.
[83] ZHANG C, MIN X, ZHANG J, et al. Reductive clean leaching process of cadmium from hydrometallurgical zinc neutral leaching residue using sulfur dioxide [J]. Journal of Cleaner Production, 2016, 113(Feb.1):910-918.
[84] DAI Z J, WANG L L, TANG H, et al. Speciation analysis and leaching behaviors of selected trace elements in spent SCR catalyst [J]. Chemosphere, 2018, 207:440-448.
[85] ALGHANMI S I, AL SULAMI A F, EL-ZAYAT T A, et al. Ac-id leaching of heavy metals from contaminated soil collected from Jeddah, Saudi Arabia:kinetic and thermodynamics studies [J]. International Soil and Water Conservation Research, 2015, 3(3):196-208.
[86] 刘淑琴, 马伟平. 富氧地下气化灰渣中有害微量元素的浸出行为[J]. 煤炭学报, 2020,45(12):4201-4208.
LIU Shuqin, MA Weiping. Leaching characteristics of hazardous trace elements in the slag from oxygen-enriched underground coal gasification [J]. Journal of China Coal Society, 2020, 45(12):4201-4208.
[87] YU Shilin, TAO Runze, TAN Houzhang, et al. Migration characteristics and ecological risk assessment of heavy metals in ash from sewage sludge co-combustion in coal-fired power plants [J]. Fuel, 2023, 333:126420.
[88] WANG Jianbin, LIU Jianzhong, LI Dedi, et al. Geochemical distribution and mineralogy of heavy metals in the gasification residue of coal-waste activated carbon-slurry:Insights into leaching behavior [J]. Journal of Hazardous Materials, 2023, 451:131146.
[89] 刘艳芳, 崔龙鹏, 郎子轩, 等. Shell粉煤气化灰渣环境风险评价[J]. 石油学报(石油加工), 2022, 38(1):137-145.
LIU Yanfang, CUI Longpeng, LANG Zixuan, et al. Environmental risk assessment of ash and slag from shell pulverized coal gasification [J]. Acta Petrolei Sinica(Petroleum Processing Section), 2022, 38(1):137-145.
[90] HUFFMAN G P, HUGGINS F E, SHAH N, et al. Speciation of arsenic and chromium in coal and combustion ash by XAFS spectroscopy [J]. Fuel Processing Technology, 1994, 39(1/3):47-62.
[91] IZQUIERDO M, QUEROL X. Leaching behaviour of elements fr-om coal combustion fly ash:An overview [J]. International Journal of Coal Geology, 2012, 94:54-66.
[92] NIU M F, FU Y G, LIU S Q. Distribution and leachability of hazardous trace elements in Lurgi gasification ash from a Coal - to - SNG plant [J]. Journal of The Energy Institute, 2021, 98:223-233.
[93] TANG Y L, ZHAO J T, ZHOU J F, et al. Highly efficient removal of Cr(III)-poly(acrylic acid) complex by coprecipitation with polyvalent metal ions:Performance, mechanism, and validation [J]. Water Research, 2020, 178:115807.
[94] QU J, ZHANG J, LI H, et al. Occurrence, leaching behavior, and detoxification of heavy metal Cr in coal gasification slag [J]. Chinese Journal of Chemical Engineering, 2023, 58:11-19.
[95] MA W P, LIU S Q, LI Z, et al. Release and transformati-on mechanisms of hazardous trace elements in the ash and slag during underground coal gasification [J]. Fuel, 2020, 281:118774.
[96] LANGE C N, FLUES M, HIROMOTO G, et al. Long-term leaching of As, Cd, Mo, Pb, and Zn from coal fly ash in column test [J]. Environmental Monitoring and Assessment, 2019, 191:602.
[97] LIEBERMAN R N, QUEROL X, MORENO N, et al. Physical and chemical changes in coal fly ash during acidic or neutral wastes treatment, and its′effect on the fixation process [J]. Fuel, 2016, 184:69-80.
[98] NEUPANE G, DONAHOE R J. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests [J]. Fuel, 2013, 104:758-770.
[99] JONES D R. The leaching of major and trace elements from coal ash[J]. Fuel &Energy Abstracts, 1995, 38(3):221-262.
[100] CORNELIS G, JOHNSON C A, VAN GERVEN T, et al. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes:A review [J]. Applied Geochemistry, 2008, 23(5):955-976.
[101] TANG Y G, GUO X, XIE Q, et al. Petrological characteristics and trace element partitioning of gasification residues from slagging entrained-flow gasifiers in Ningdong, China [J].Energy &Fuels, 2018, 32(3):3052-3067.
[102] WANG N N, SUN X Y, ZHAO Q, et al. Leachability and adverse effects of coal fly ash:A review [J]. Journal of Hazardous Materials, 2020, 396:122725.
[103] ZHAO L, DAI S F, FINKELMAN R B, et al. Leaching behav-ior of trace elements from fly ashes of five Chinese coal power plants [J]. International Journal of Coal Geology, 2020, 219:103381.
[104] GUO Y, LI H, QIU G, et al. Processing of coal gasification fine slag by different physical separation methods:Fate of typical heavy metals and comparison analysis on products [J]. Separation and Purification Technology, 2023, 306:122675.
[105] 刘雪梅, 罗思梦. 固化稳定化技术对垃圾焚烧飞灰中重金属的研究[J]. 应用化工, 2022(3):816-820.
LIU Xuemei, LUO Simeng. Study on heavy metals in MSWI fly ash by solidification stabilization technology[J]. Applied Chemical Industry, 2022(3):816-820.
[106] 朱菊芬, 李健, 闫龙, 等 煤气化渣资源化利用研究进展及应用展望[J]. 洁净煤技术, 2021, 27(6):11-21.
ZHU Jufen, LI Jian, YAN Long, et al. Research progress and application prospect of coal gasification slag resource utilization [J]. C1ean Coal Technology, 2021,27(6):11-21.
[107] XUE Y, LIU X M. Detoxification, solidification and recycling of municipal solid waste incineration fly ash:A review [J]. Chemical Engineering Journal, 2021, 420:130349.
[108] BIE R S, CHEN P, SONG X F, et al. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment [J].Journal of The Energy Institute, 2016, 89(4):704-712.
[109] MAO Y P, WU H, WANG W L, et al. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material [J].Journal of Hazardous Materials, 2020, 385:121580.
[110] BAO J P, WANG L, XIAO M. Changes in speciation and leaching behaviors of heavy metals in dredged sediment solidified/stabilized with various materials [J]. Environmental Science and Pollution Research, 2016, 23(9):8294-8301.
[111] OUHADI V R, YONG R N, DEIRANLOU M. Enhancement of cement-based solidification/stabilization of a lead-contaminated smectite clay [J]. Journal of Hazardous Materials, 2021, 403:123969.
[112] 史兆臣, 戴高峰, 王学斌, 等. 煤气化细渣的资源化综合利用技术研究进展[J]. 华电技术, 2020, 7:63-73.
SHI Zhaochen, DAI Gaofeng, WANG Xuebin, et al. Review on the comprehensive resources utilization technology of coal gasification fine slag [J].Huadian Technology, 2020, 7:63-73.
[113] ZHANG Y Y, WANG L, CHEN L, et al. Treatment of municipal solid waste incineration fly ash:State-of-the-art technologies and future perspectives [J]. Journal of Hazardous Materials, 2021, 411:125132.
[114] AINETO M, ACOSTA A, IGLESIAS I. The role of a coal gasification fly ash as clay additive in building ceramic [J]. Journal of the European Ceramic Society, 2006, 26(16):3783-3787.
[115] MA W C, SHI W B, SHI Y J, et al. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash [J]. Journal of Hazardous Materials, 2021, 408:124809.
[116] 张凯, 刘舒豪, 张日新, 等. 免烧法煤气化粗渣制备陶粒工艺及其性能研究[J]. 煤炭科学技术, 2018,46 (10):222-227.
ZHANG Kai, LIU Shuhao, ZHANG Rixin, et al. Research on preparation of non-sintered ceramsite from gasification cinder and its performance [J]. Coal Science and Technology, 2018, 46(10):222-227.
[117] OGATA F, IWATA Y, KAWASAKI N. Properties of novel adsorbent produced by hydrothermal treatment of waste fly ash in alkaline solution and its capability for adsorption of tungsten from aqueous solution [J]. Journal of Environmental Chemical Engineering, 2015, 3(1):333-338.
[118] HUANG H J, YUAN X Z. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge [J]. Bioresource Technology, 2016, 200:991-998.
[119] SHAO J G, YUAN X Z, LENG L J, et al. The comparison of the migration and transformation behavior of heavy metals during pyrolysis and liquefaction of municipal sewage sludge, paper mill sludge, and slaughterhouse sludge [J]. Bioresource Technology, 2015, 198:16-22.
[120] CHEN Z, YU G W, ZOU X Y, et al. Co-disposal of incineration fly ash and sewage sludge via hydrothermal treatment combined with pyrolysis:Cl removal and PCDD/F detoxification [J]. Chemosphere, 2020, 260:127632.
[121] LIANG Y J, CHAI L Y, MIN X B, et al. Hydrothermal sulfidation and floatation treatment of heavy-metal-containing sludge for recovery and stabilization [J]. Journal of Hazardous Materials, 2012, 217:307-314.
[122] MIN X B, YUAN C Y, LIANG Y J, et al. Metal recovery from sludge through the combination of hydrothermal sulfidation and flotation [J]. Procedia Environmental Sciences, 2012, 16:401-408.
[123] ZHAN L, JIANG L, ZHANG Y L, et al. Reduction, detoxification and recycling of solid waste by hydrothermal technology:A review [J]. Chemical Engineering Journal, 2020, 390:124651.
[124] 胡艳军, 方祝敏, 严密, 等. 水热法稳定垃圾焚烧飞灰重金属研究[J]. 浙江工业大学学报, 2018(2):192-198.
HU Yanjun, FANG Zhumin, YAN M, et al. Research on stabilization of heavy metals in MSWI fly ash by hydrothermal treatement [J]. Journal of Zhejiang University of Technology, 2018(2):192-198.
[125] WDOWIN M, FRANUS W, FRANUS M. Hydrothermal techno-
logy of zeolite materials synthesis from fly ash[J]. International Journal of Arts &Sciences, 2013, 6(3):391-396.
[126] GOUGAZEH M, BUHL J C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin [J]. Journal of the Association of Arab Universities for Basic &Applied Sciences, 2014, 15(1):35-42.
[127] JING Z, JIN F, HASHIDA T, et al.Hydrothermal solidification of blast furnace slag by formation of tobermorite [J]. Journal Of Materials Science, 2007, 42(19):8236-8241.
[128] YIN J, LI G M, HE W Z, et al. Hydrothermal decomposition of brominated epoxy resin in waste printed circuit boards [J].Journal of Analytical and Applied Pyrolysis, 2011, 92(1):131-136.
[129] 陈志良. 机械化学法降解垃圾焚烧飞灰中二噁英及协同稳定化重金属的机理研究[D]. 杭州:浙江大学, 2019.
[130] LI X, CHEN Q, ZHOU Y, et al. Stabilization of heavy metals in MSWI fly ash using silica fume [J]. Waste Manage, 2014, 34(12):2494-2504.
[131] 黎雯, 王文祥, 方红生, 等. 硅铝添加剂对机械化学法稳定飞灰中重金属的影响机制研究[J]. 无机盐工业, 2023(4):84-91.
LI Wen, WANG Wenxiang, FANG Hongsheng, et al. Study on effect mechanism of silicon-aluminum additives on stabilization of heavy metals in fly ash by mechanochemical stabilization method [J]. Inorganic Chemicals Industry, 2023(4):84-91.
[132] JIANG Q, HE Y, WU Y, et al. Solidification/stabilization of soil heavy metals by alkaline industrial wastes:A critical review [J]. Environmental Pollution, 2022, 312:120094.
[133] ZHA F S, WANG H, XU L, et al. Initial feasibility study in adsorption capacity and mechanism of soda residue on lead (II):Contaminated soil in solidification/stabilization technology [J]. Environmental Earth Sciences, 2020, 79(10):230.
[134] YANG L Y, WEI T C, LI S W, et al. Immobilization pers-istence of Cu, Cr, Pb, Zn ions by the addition of steel slag in acidic contaminated mine soil [J].Journal of Hazardous Materials, 2021, 412:125176.
[135] NING D F, LIANG Y C, LIU Z D, et al. Impacts of steel-sl-ag-based silicate fertilizer on soil acidity and silicon availability and metals-immobilization in a paddy soil[J]. Plos One, 2016, 11(12):e0168163.
[136] BASHIR S, SALAM A, REHMAN M, et al. Effective role of biochar, zeolite and steel slag on leaching behavior of cd and its fractionations in soil column study[J]. Bulletin of Environmental Contamination and Toxicology, 2019, 102(4):567-572.
[137] WANG X B, YAN X, LI X Y. Environmental risk for applica-tion of ammonia-soda white mud in soils in China [J].Journal of Integrative Agriculture, 2020, 19(3):601-611.
[138] LIU W, YU Y X. Removal of recalcitrant trivalent chromium complexes from industrial wastewater under strict discharge standards [J]. Environmental Technology &Innovation, 2021, 23:101644.
[139] ISHFAQ A, ILYAS S, YASEEN A, et al. Hydrometallurgical valorization of chromium, iron, and zinc from an electroplating effluent [J]. Separation and Purification Technology, 2019, 209:964-971.
[140] 倪鹏. 垃圾焚烧飞灰矿化解毒一体化的研究[D]. 武汉:华中科技大学, 2018.
[141] LIU W, TENG L, ROHANI S, et al. CO2 mineral carbonation using industrial solid wastes:A review of recent developments [J]. Chemical Engineering Journal, 2021, 416:129093.
[142] 武鸽, 刘艳芳, 崔龙鹏, 等. 典型工业固体废物碳酸化反应性能的比较[J]. 石油学报(石油加工), 2020,36(1):169-178.
WU Ge, LIU Yanfang, CUI Longhai, et al. Comparison of the carbonation reaction properties of typical industrial solid wastes[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020,36(1):169-178.
[143] NI P, XIONG Z, TIAN C, et al. Influence of carbonation under oxy-fuel combustion flue gas on the leachability of heavy metals in MSWI fly ash [J]. Waste Manage, 2017, 67:171-180.
[144] JIANG J G, CHEN M Z, ZHANG Y, et al. Pb stabilization in fresh fly ash from municipal solid waste incinerator using accelerated carbonation technology [J]. Journal of Hazardous Materials, 2009, 161(2/3):1046-1051.
[145] 杨刚. 粉煤灰矿化封存CO2协同重金属固化[D]. 北京:华北电力大学, 2021.
[146] JIANG J G, TIAN S C, ZHANG C. Influence of SO2 in incineration flue gas on the sequestration of CO2 by municipal solid waste incinerator fly ash [J]. Journal of Environmental Sciences, 2013, 25(4):735-40.
[147] 龚泽儒,王晓娜,吕俊复,等. 等离子体煤气化技术研究进展[J]. 洁净煤技术, 2019, 25(1):35-40.
GONG Zeru, WANG Xiaona, LYU Junfu, et al. Research on plasma gasification technology of coal [J]. Clean Coal Technology, 2019, 25(1):35-40.
Migration pattern and solidification treatment technology of heavy metals in coal gasification slag:A review