碳酸酐酶固定及在二氧化碳捕集应用研究进展
Research progress on the immobilization of carbonic anhydrase and its application in carbon dioxide capture
0 引 言
化石燃料如煤、石油和天然气的使用向大气中排放大量二氧化碳是导致气温上升、海平面变化和极端天气等现象发生的主要原因。但受能源供应与需求的影响,目前主要能源仍是化石能源[1]。因此,减少因使用化石燃料产生的二氧化碳排放至关重要。二氧化碳捕集、利用及封存(CCUS)技术在减少碳排放的同时,还可将CO2转化为有价值的产品,在控制全球气候变化、维持经济可持续性发展方面发挥了重要作用[2]。
目前CO2捕集技术主要有物理/化学吸收法、吸附法、膜吸收法和生物固定法等[3]。其中,利用碱性液体吸收剂将CO2直接从烟气中分离的化学吸收法,具有分离效率高、成本低等优点,是目前最成熟的工艺[4]。但存在解吸过程中消耗能量较多、溶剂长期使用会产生降解并造成设备腐蚀性等问题[5]。碳酸酐酶(Carbonic anhydrase,CA)是一种高效的CO2水合催化剂。将CA应用在CCUS技术中,可以提高CO2的吸收效率,有效解决传统工艺中的热能损失,逐渐成为二氧化碳捕集与封存研究中的热点。CA捕集CO2技术具有环境友好、能耗低、可再生、无二次污染等优点[6]。
CA是一种以Zn2 为活性中心的金属酶,具有巨大的生物催化潜力。目前,利用碳酸酐酶法捕集CO2的主要机理是利用碳酸酐酶催化CO2水合生成碳酸氢根后,在Ca2 、Mg2 等金属离子的参与下将CO2以碳酸盐形式固定下来。然而,CA成本较高、游离CA在高温和废气含量大等恶劣条件下稳定性差、可重用性差,限制了其工业应用。固定化酶技术是利用物理或化学手段解决上述问题的有效方法之一。将游离酶封锁在载体材料内或限制在一定区域内进行催化作用的一种技术。CA固定化保持了游离CA反应条件温和、高效专一等特性,同时又弥补了游离CA的不足,具备储存稳定性高、易于分离回收、可重复使用、连续操作可控、工艺设备简便等优点,固定化技术扩大了酶在实际生产中的应用范围[7]。常用的载体材料可分为高分子材料、无机材料、聚合物-无机复合材料等。根据使用场合和要求来选择合适的载体材料和固定化方法对于获得理想性能的固定化CA至关重要[8]。
本文介绍了碳酸酐酶的固定方法和载体材料以及碳酸酐酶在二氧化碳捕集中的作用机理,总结了碳酸酐酶在促进化学溶剂吸收二氧化碳和诱导二氧化碳矿化生成碳酸钙的最新研究进展。
1 碳酸酐酶的固定化
CA是已知催化CO2水合速率最快的酶,其催化吸收速率可达106 s-1。在有机胺溶液中加入CA可以显著降低解析时需要的温度,大大减少再生所需能耗[9]。由于CA自身的热稳定性不高和重复利用率较低,限制了其大规模应用,因此,分子修饰、定向进化和酶固定化等被用来提高其稳定性和活性[10]。化学修饰分为残基特异性修饰和基团特异性修饰。徐霞等[11]将Bacillus Clausii中CA上的29位天冬氨酸、233位甘氨酸或83位天冬氨酸进行突变,构建含有目的基因的载体,有效提高了CA在高温条件下的CO2捕集转化效率。Alvizo等[12]利用定向进化技术,筛选出高度稳定的CA变异体,在pH>10.0的4.2 mol/L碱性胺溶剂下,该变异体能耐受高达107 ℃的温度,极大提高了普通脱硫弧菌β类CA的性质。虽然前2种方法可以提高CA对高温、极端pH值等极端操作条件的抵抗力,但需通过固定化来提高CA的可重用性,且酶固定化具有方便、直接等优点,常被作为提高CA稳定性和活性的技术手段之一[13]。
1.1 碳酸酐酶的固定方法
由于酶易变性和失活,合理的固定化可以提高酶的刚性和稳定性,以克服其在实际应用中的局限性。通常酶固定化方法包括物理法和化学法。物理法分为吸附法和包埋法,化学法分为共价结合法和交联法[14]。4种酶固定方法如图1所示。吸附法是最简单的酶固定方法,包括物理吸附和离子交换吸附。吸附法利用载体和酶之间的物理相互作用力(如范德华力、离子键合、氢键、电荷转移、亲疏水性等)将酶固定在载体表面[15]。包埋法又可以分为凝胶包埋法和微囊化包埋法。凝胶包埋将酶限制在高聚物网格中,而微囊化包埋则将酶置于不同构型的膜外壳内。共价结合法是指酶蛋白分子的功能基团与载体表面基团之间以共价键结合的一种固定化方法。因此,以共价结合法制备生物复合酶时,需对载体进行改性,使其表面附着氨基、羧基、羟基、醛基等功能性基团[16]。交联法将酶分子和双功能或多功能试剂分子之间以共价键结合,再与载体材料进一步结合。4种固定化方法的优缺点见表1,酶的固定化方法要根据酶的应用领域选择。

图1 CA固定方法[17]
Fig.1 Immobilization methods of carbonic anhydrase[17]
表1 CA固定方法优缺点
Table 1 Advantages and disadvantages of CA immobilization methods

1.2 碳酸酐酶固定化载体
载体的选择会显著影响固定化酶的性质,制备理想的固定化酶既要选用合理有效的固定化方法,还要选择良好的载体。通常在选择固定化载体材料时需考虑以下几点:① 载体的理化性质,如形状、大小、孔径、机械强度、不易溶于反应介质、强酸碱和高温条件下的稳定性、耐微生物降解能力等;② 载体的工业应用能力,材料价廉易得、可进行工业化大生产、可再生循环使用等[21]。
传统的固定化载体主要是以动植物结构蛋白为主的天然高分子产物,如从虾的甲壳中提取的壳聚糖、甲壳素;从海藻中可以提取海藻酸钠等。此外还包括琼脂糖珠、琼脂糖凝聚、卵清蛋白、木质纤维等[22]。近几年,包括碳基材料、硅基材料、金属氧化物等在内的无机载体材料研究较多,且大量研究利用化学试剂对材料改性后再用于固定CA,使固定化CA的稳定性、可重复利用性更好。固定化CA的种类及其载体见表2。牛建杰等[23]以γ-缩水甘油醚氧丙基三甲氧基硅烷(GPTMS)为改性剂对纳米颗粒进行改性,得到了环氧基功能化的Fe3O4-SiO2核壳纳米颗粒,随后将碳酸酐酶CA共价固定到纳米颗粒上,以实现CA固定化。结果表明,固定化酶的热稳定性、贮藏稳定性和循环使用性均优于同等条件下的游离酶,其在循环使用10次后仍保持84.2%的相对酶活力。Jing等[24]成功将CA固定在磁性聚微球上,与游离CA相比,固定化CA具有更好的酶活性、较好的可重用性、更高的热稳定性。经过6次重复使用后,活性为最初的47.6%。Kim等[25]将大肠杆菌核糖体蛋白L2与海洋细菌海洋氢基因弧菌(hmCA)的CA基因融合,构建的融合蛋白成功自固定到硅藻生物硅,制备的固定化酶具有较高的稳定性。Peirce等[26]在存在碳化二亚胺的条件下,通过共价键将耐热的CA固定在顺磁性Fe3O4纳米粒子(NPs)上,最大载酶量为40 mg/g(以NPs计)。Kumari等[27]将Sulfurihydrogenibium azorense CA(SazCA)与纤维素结合模块(CBM3)融合后得到的重组蛋白BMC-SazCA可与微晶纤维素珠子紧密结合,将固定化酶应用于催化CO2水合反应,10个循环后,固定化BMC-SazCA的活性约为新鲜制备固定化BMC-SazCA的90%。
表2 固定化CA载体
Table 2 Immobilized carriers of carbonic anhydrase

2 碳酸酐酶在二氧化碳捕集中的应用
2.1 对化学溶剂吸收二氧化碳的促进作用
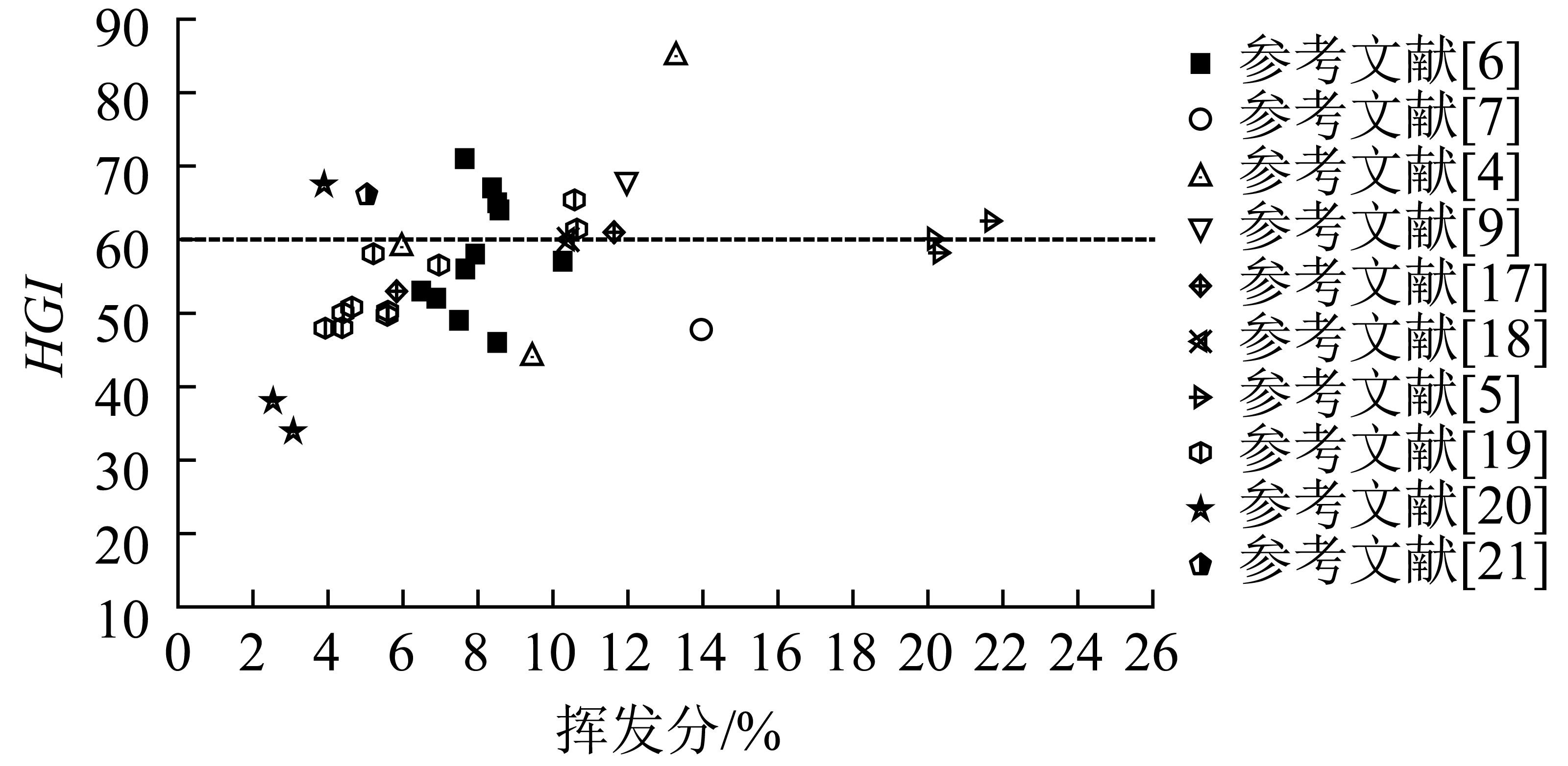
利用化学吸收法捕集二氧化碳常用的化学溶剂为醇胺和金属碱性试剂[37]。醇胺溶液包括一乙醇胺(MEA)、二乙醇胺(DGA)、哌嗪(PZ)、二乙烯三胺(DETA)、甲基二乙醇胺(MDEA)和五甲基二乙烯三胺(PMDETA)等[38]。金属碱性试剂有碳酸钾、氢氧化钠、氢氧化镁等。利用醇胺溶液吸收CO2具有较大的吸收容量,易解吸、腐蚀低,但产物易降解,吸收焓较高,吸收速率有待提升。利用碳酸钾(K2CO3)溶液吸收CO2需要较低的再生能量,对环境更友好,但其吸收动力学较慢,因此需要大型且昂贵的吸收柱才能操作。碳酸酐酶稳定性好,在加快CO2水合速率的同时又可以降低吸收焓。因此,大量研究表明在醇胺或金属碱性试剂中加入碳酸酐酶,可以使CO2捕集工艺同时具有高吸收容量、较快吸收速率和较低解吸能耗[39]。
Silverman等[40]探讨了碳酸酐酶的结构和催化机理,结果表明,尽管不同类型的CA具有不同的蛋白质序列,但催化的活性中心都是带羟基的Zn2 离子,CA的加入可明显提高CO2水合速率。CA催化水合反应机理如图2所示。在分子间质子转移中,CA处于活性状态,氢氧离子结合到锌上,二氧化碳分子靠近氨基酸侧链,这里称为质子通道,向溶液中的缓冲分子释放质子。然后结合锌的羟基对碳原子进行亲核攻击与附近的二氧化碳分子反应,产生结合锌的碳酸氢盐。水分子将碳酸氢盐交换到溶液后,酶处于不活跃状态,水与锌结合。为了恢复CA的催化活性,必须从与锌结合的水分子中去掉一个质子。质子通过质子通道完成分子内质子转移,这种转移发生在氢键水分子之间[41]。

图2 CA催化水合反应的机理[42]
Fig.2 Mechanism of CA catalyzed hydration reaction[42]
使用MEA溶剂吸收CO2是迄今为止捕集CO2最成熟的技术。但MEA溶剂仍存在再生能量高、溶剂降解、挥发性大、毒性大等问题[43]。Feron[44]认为可形成碳酸氢盐的溶剂(如叔胺和碱性碳酸盐)是一种很有前途的替代物。Gladis等[45]探究了碳酸酐酶对MEA、2-氨基-2-甲基-1-丙醇(AMP)、MDEA和K2CO3溶剂吸收CO2能力的影响。研究表明,CA的加入对各溶剂液相传质系数的影响依次为:MDEA显著提高,K2CO3和AMP略有增加,而MEA基本不变。说明CA对于可形成碳酸氢盐溶剂的催化效果更好。Leimbrink等[46]将包埋在有机硅基聚合物基质中的CA加入MDEA水溶液后,与空白MDEA溶剂相比,在固定化CA的存在下,CO2总吸收摩尔流量增加了6倍。Gladis等[47]研究发现,CA的加入对提高MDEA溶剂CO2捕集率有积极影响,增加酶的添加量可进一步提高捕集效率。中试吸收试验表明,CA的加入对CO2传质速率有明显促进作用,30%的MDEA溶剂在10 m柱高时可捕集18%~23%CO2,在相同液气比范围内,加入0.85 g/L CA后的CO2捕集率在36%~49%。当CA浓度增加到3.5 g/L时,捕集效率高达48%~83%。Ai等[48]将固定在环氧磁性复合微球上的CA酶加到10%的MDEA溶液中,CO2吸收速率比原MDEA溶液提高近40%,反应平衡时间从150 min缩短到90 min,经7次循环使用后,固定化CA的活性在313.15 K时仍接近其初始值。刘彬等[49]研究发现牛碳酸酐酶(BCA)的加入可显著提高MDEA吸收CO2的反应速率,且反应速率随BCA浓度的增加而增大。CA在二氧化碳捕集中的催化性能见表3。
表3 CA在二氧化碳捕集中的催化性能
Table 3 Catalytic performance of CA in carbon dioxide capture

K2CO3溶液使用时需要较低的再生能量,更环保,但吸收动力学较慢,因此需要较大且昂贵的吸收塔才能操作。这一工业限制可以向K2CO3溶液中添加CA等促进剂来解决。Hu等[54]用湿壁柱测定了323 K下,耐热CA在30% K2CO3溶液(pH=11~12)中的催化系数为5.3×108 M-1s-1。耐热CA在pH=10.6~10.8、323 K的30% K2CO3溶液中连续运行8 h后较稳定,初始催化效率仍保持在70%以上。Qi等[52]在吸收塔温度40 ℃、汽提塔压力35 kPa条件下,在23.5% K2CO3溶剂中加入2.5 g/L的CA,集成小试系统成功运行500 h后,CO2平均捕集率为84%。通过湿壁柱试验表明,当加入2 g/L CA时,CO2传质系数可显著提高5倍。
CA作为化学溶液吸收CO2的活化剂还有问题需要解决。首先,CA作用机理非常复杂,需进一步开发检测新技术,深入理解其作用机制,从本质上分析催化过程的理论,推导出更准确的催化机理。此外,需评估用于CO2捕集过程的酶促进剂:包括酶的温度稳定性、相分离特性和寿命,以及不同浓度配比对CA催化吸收速率的影响,以便能够可靠设计混合、分离、回收和其他辅助步骤,以优化吸收系统性能[39]。烟气中存在的SOx和NOx能抑制CA[55]。SO2溶于水中会形成亚硫酸,通常在催化剂如NO2存在下,SO2可进一步被氧化生成硫酸,故烟气中SOx和NOx会以 和
和![]() 等形式存在于吸收液中,对CA产生影响。Bond等[56]研究了
等形式存在于吸收液中,对CA产生影响。Bond等[56]研究了![]() 和
和![]() 对牛CA的影响,结果表明,
对牛CA的影响,结果表明,![]() 浓度为5 mmol/L时几乎没有抑制作用,但浓度达到50 mmol/L时有显著抑制作用;
浓度为5 mmol/L时几乎没有抑制作用,但浓度达到50 mmol/L时有显著抑制作用;![]() 浓度在5~50 mmol/L对CA有明显的抑制作用。李娟等[57]发现吸收液的pH随SO2浓度的增大而降低,固定化CA的活性受到抑制,SO2存在不利于CA对CO2的催化吸收。Faridi等[58]研究发现
浓度在5~50 mmol/L对CA有明显的抑制作用。李娟等[57]发现吸收液的pH随SO2浓度的增大而降低,固定化CA的活性受到抑制,SO2存在不利于CA对CO2的催化吸收。Faridi等[58]研究发现![]() 浓度为1 mol/L 时可以激活重组
浓度为1 mol/L 时可以激活重组 在1 mol/L时也不影响重组α-CA的活性,
在1 mol/L时也不影响重组α-CA的活性,![]() 可增强重组α-CA的活性和热稳定性。Ramanan等[59]发现
可增强重组α-CA的活性和热稳定性。Ramanan等[59]发现![]() 对来自菌株Citrobacter freundii的CA也有激活作用。不同来源的CA对烟道气中存在的SOx和NOx的耐受能力不同,因此CA对所处理烟道气有一定要求,牛CA处理的应是经脱硫脱氮处理的烟道气。故未来需继续探究不同来源的CA受烟道气中组分的影响,以更好地应用于现场碳捕集。
对来自菌株Citrobacter freundii的CA也有激活作用。不同来源的CA对烟道气中存在的SOx和NOx的耐受能力不同,因此CA对所处理烟道气有一定要求,牛CA处理的应是经脱硫脱氮处理的烟道气。故未来需继续探究不同来源的CA受烟道气中组分的影响,以更好地应用于现场碳捕集。
2.2 对二氧化碳矿化生成碳酸钙的诱导作用
随着碳浓缩机制的发展,一些细菌可以将高含量CO2转化为碳酸钙(CaCO3)、生物燃料和生物表面活性剂等高附加值产品。CA将CO2通过生物矿化固定为CaCO3的过程中发挥了重要作用。利用CA诱导矿化是一种高效、稳定、生态友好且可长期储存CO2的方法[60]。Li等[61]研究表明CA可以加快CaCO3的沉淀速率,CaCO3主要晶相为方解石。Cizer等[62]研究表明CA有利于形成稳定的CaCO3,并通过形成新的晶面显著改变其形态。CA诱导CaCO3沉淀的实质是催化水中Ca2 与CO2的反应[63]。微生物产CA诱导CaCO3沉淀机理如图3所示,反应方程[64]为
1)气态二氧化碳溶解于水中,生成水合二氧化碳:
CO2(g)![]() CO2(aq),
CO2(aq),
(1)
2)水合二氧化碳与水反应生成H2CO3:
CO2(aq) H2O![]() H2CO3,
H2CO3,
(2)
3)H2CO3在水中电离生成H 和

(3)
4)在碱性条件下,![]() 电离形成
电离形成![]() 和H2O:
和H2O:

(4)
5)在Ca2 存在的情况下,CaCO3与![]() 反应生成CaCO3沉淀:
反应生成CaCO3沉淀:

(5)

图3 微生物诱导碳酸钙在溶液中沉淀的机理模型[64]
Fig.3 Mechanism model of microbial induced calcium carbonate precipitation in the solution[64]
上述反应中影响CaCO3生成的限速步骤主要是CO2和水反应生成碳酸氢盐。自然界中,CO2水合反应速率相当慢,转化系数仅为1.3×10-1 s-1,CA存在可以加速上述反应,CO2水合物转化系数显著提高到1.0×106 s-1,是自然界的107倍。因此,CA加速CO2的水合反应是CaCO3沉淀的主导因素。
在CA诱导矿化过程中,CA活性受各种因素影响,如温度、酸碱度和Ca2 浓度。因此,研究不同因素对CA活性的影响,进一步阐明CA促进CaCO3矿化的机理具有重要意义。Li等[65]研究了不同Ca2 浓度下CA催化CaCO3沉淀的动力学。CaCO3沉淀速率随Ca2 浓度的增加而增加,但Ca2 浓度高于100 mmol/L时对CA催化CaCO3沉淀有负面影响。Li等[66]研究了CA在初始pH分别为6.0、6.5、7.0和8.0时促进CaCO3沉淀的动力学。结果表明,初始pH=8.0时,体系中Ca2 离子在48 h内完全沉淀,分别比初始pH为6.0、6.5和7.0时提前21、15和14 h,说明在试验pH范围内,较高的pH值有利于CA催化CaCO3沉淀。袁亮[67]研究了温度、pH值和Ca2 浓度对细菌生长、CA活性的影响。结果表明,25 ℃时CA活性最高,有利于CaCO3沉淀;初始pH值为8.5时,CaCO3沉淀最多;Ca2 浓度为50 mmol/L时,细菌生长繁殖最好,过低的Ca2 浓度会影响CaCO3生成,而过高的Ca2 浓度则会严重影响细菌生长,降低细菌活性。
3 结语与展望
碳酸酐酶(carbonic anhydrase,CA)是一种以Zn2 为活性中心的金属酶,具有巨大的生物催化潜力。但由于CA自身的热稳定性不高和重复利用率较低,限制了其大规模应用,因此,分子修饰、定向进化和酶固定化等被用来提高其稳定性和活性。近几年研究方向逐渐将这几种策略结合起来,如将分子修饰后的CA进行固定化应用于CO2捕集。固定化载体材料不仅具有较高的机械和化学稳定性,还要避免外界因素导致CA浸出,更重要的是,这种新型材料的制备条件应简单温和,很好地保留固定化过程中CA的活性。此外,载体材料应与底物有足够的亲和力,以增强CO2的捕集和传质。虽然CA可以通过CO2的水合作用促进碳的捕集和分离过程,但离实际应用还有差距。对此,可从以下方面进行研究:
1)开发具有更高活性和稳定性的新型CA。利用基因克隆、蛋白质工程和合成生物学等理论,可以设计出对各种极端环境适应性强的游离CA。
2)廉价高性能载体材料的开发。载体材料应具有较高的机械和化学稳定性,以抑制CA浸出、避免外界侵袭。此外,新型材料的制备条件应简单温和,以很好地保留固定化过程中CA的活性。
3)进一步探究CA内在因素和外界条件对其工业应用产生的阻碍,如酶的成本、批量生产、热稳定性、贮藏稳定性、催化寿命以及其对SOx和NOx等烟道气成分的耐受性等问题。
[1] WITHEY P,JOHNSTON C,GUO J. Quantifying the global warming potential of carbon dioxide emissions from bioenergy with carbon capture and storage[J]. Renewable and Sustainable Energy Reviews,2019,115:109408.
[2] K R
R SZOV
SZOV M,ZACH B,PETRUSOV
M,ZACH B,PETRUSOV Z,et al. Post-combustion carbon capture by membrane separation,Review[J]. Separation and Purification Technology,2020,238:116448.
Z,et al. Post-combustion carbon capture by membrane separation,Review[J]. Separation and Purification Technology,2020,238:116448.
[3] SONG C,LIU Q,DENG S,et al. Cryogenic-based CO2 capture technologies:State-of-the-art developments and current challenges[J]. Renewable and Sustainable Energy Reviews,2019,101:265-278.
[4] ZHANG X,HUANG Y,YANG J,et al. Amine-based CO2 capture aided by acid-basic bifunctional catalyst:Advancement of amine regeneration using metal modified MCM-41[J]. Chemical Engineering Journal,2020,383:123077.
[5] MAZARI S A,GHALIB L,SATTAR A,et al. Review of modelling and simulation strategies for evaluating corrosive behavior of aqueous amine systems for CO2 capture[J]. International Journal of Greenhouse Gas Control,2020,96:103010.
[6] FROST S C,MCKENNA R. Carbonic anhydrase:mechanism,regulation,links to disease,and industrial applications [M]. Dordrecht:Springer Science & Business Media,2013.
[7] 费潇瑶. 碳酸酐酶的固定化及其CO2的捕集性能[D].大连:大连理工大学,2018.
FEI Xiaoyao. Immobilization ofcarbonic anhydrase and the performance in CO2 capture[D]. Dalian:Dalian University of Technology,2018.
[8] 刘文芳,魏利娜. 碳酸酐酶固定化研究进展[J]. 分子催化,2016,30(2):182-97.
LIU Wenfang,WEI Li′na. Research progress on carbonic Anhydrase Immobilization[J]. Journal of Molecular Catalysis(China),2016,30(2):182-197.
[9] 张士汉,沈遥,杜敏娥,等. 一种固定化碳酸酐酶及其制备与在捕集烟气中二氧化碳的应用:CN109517816A[P].2019-03-26.
ZHANG Shihan,SHEN Yao,DU Mine,et al. An immobilized carbonic anhydrase and its preparation and application in the capture of carbon dioxide from flue gas:CN109517816A[P].2019-03-26.
[10] REN S,JIANG S,YAN X,et al. Challenges and opportunities:Porous supports in carbonic anhydrase immobilization[J]. Journal of CO2 Utilization,2020,42:101305.
[11] 徐霞,陈金瑞,马亮,等. 一种高活性碳酸酐酶及构建方法和应用:CN108374005A[P].2018-08-07.
XU Xia,CHEN Jinrui,MA Liang,et al. The invention relates to a highly activated carbonic anhydrase and its construction method and application:CN108374005A[P].2018-08-07.
[12] ALVIZO O,NGUYEN L J,SAVILE C K,et al. Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas[J]. Proceedings of the National Academy of Sciences,2014,111(46):16436-16441.
[13] CUI J D,JIA S R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology:Current development and future challenges[J]. Critical Reviews in Biotechnology,2015,35(1):15-28.
[14] LIU D M,CHEN J,SHI Y P. Advances on methods and easy separated support materials for enzymes immobilization[J]. TrAC Trends in Analytical Chemistry,2018,102:332-342.
[15] GENNARI A,FÜHR A J,VOLPATO G,et al. Magnetic cellulose: Versatile support for enzyme immobilization-A review[J]. Carbohydrate Polymers,2020,246:116646.
[16] 江正强,杨绍青. 食品酶学与酶工程原理[M]. 北京:中国轻工业出版社,2018:190-230.
JIANG Zhengqiang,YANG Shaoqing. Principles of food enzymology and enzyme engineering[M]. Beijing:China Light Industry Press,2018:190-230.
[17] EFFENDI S S W,NG I S. The prospective and potential of carbonic anhydrase for carbon dioxide sequestration:A critical review[J]. Process Biochemistry,2019,87:55-65.
[18] 林海蛟,张继福,张云,等. 基于大孔吸附树脂先交联后吸附法固定化脂肪酶[J]. 广西师范大学学报(自然科学版),2020,38(4):100-108.
LIN Haijiao,ZHANG Jifu,ZHANG Yun,et al. Immobilization of lipase by crosslinking and then adsorption method using macroporous adsorbent resin[J]. Journal of Guangxi Normal University(Natural Science Edition),2020,38(4):100-108.
[19] 周稳,管政兵,蔡宇杰,等. 芽胞漆酶的固定化方法对比研究与载体选择[J]. 食品与生物技术学报,2017,36(4):364-370.
ZHOU Wen,GUAN Zhengbing,CAI Yujie,et al. Immobilization of spore laccase:Comparative studies and carriers selection[J]. Journal of Food Science and Biotechnology,2017,36(4):364-370.
[20] FOPASE R,PARAMASIVAM S,KALE P,et al. Strategies,challenges and opportunities of enzyme immobilization on porous silicon for biosensing applications[J]. Journal of Environmental Chemical Engineering,2020,8(5):104266.
[21] 贾峰,郑连炳,王志强. 生物酶固定化技术研究现状 [J]. 资源节约与环保,2020(4):116.
JIA Feng,ZHENG Lianbing,WANG Zhiqiang. Research status of enzyme immobilization technology[J]. Resources Economization & Environmental Protection,2020(4):116.
[22] 刘雪凌,林贝. 载体固定化酶的应用及前景展望 [J]. 广州化工,2020,48(9):22-24.
LIU Xueling,LIN Bei. Application andprospect of carrier immobilized enzyme[J]. Guangzhou Chemical Industry,2020,48(9):22-24.
[23] 牛建杰,刘琦,张声威,等. 碳酸酐酶在功能化Fe3O4-SiO2核壳纳米颗粒上的固定及其性能表征研究[J].热力发电,2021,50(1):110-114.
NIU Jianjie,LIU Qi,ZHANG Shengwei,et al. Immobilization and characterization of carbonic anhydrase on functionalized Fe3O4-SiO2 core-shell nanoparticles[J]. Thermal Power Generation,2021,50(1):110-114.
[24] JING G,PAN F,LV B,et al. Immobilization of carbonic anhydrase on epoxy-functionalized magnetic polymer microspheres for CO2 capture[J]. Process Biochemistry,2015,50(12):2234-2241.
[25] KIM S,JOO K I,JO B H,et al. Stability-controllable self-immobilization of carbonic anhydrase fused with a silica-binding tag onto diatom biosilica for enzymatic CO2 capture and utilization[J]. ACS Applied Materials & Interfaces,2020,12(24):27055-27063.
[26] PEIRCE S,RUSSO M E,PERFETTO R,et al. Kinetic characterization of carbonic anhydrase immobilized on magnetic nanoparticles as biocatalyst for CO2 capture[J]. Biochemical Engineering Journal,2018,138:1-11.
[27] KUMARI M,LEE J,LEE D W,et al. High-level production in a plant system of a thermostable carbonic anhydrase and its immobilization on microcrystalline cellulose beads for CO2 capture[J].Plant Cell Reports,2020,39(10):1317-1329.
[28] ZHAI T,WANG C,GU F,et al. Dopamine/polyethy-lenimine mod-ified silica for enzyme immobilization and strengthening of enzymatic CO2 conversion[J]. ACS Sustainable Chemistry & Engineering,2020,8(40):15250-15257.
[29] WANG J,LYU Y. An enzyme-loaded reactor using MOF-templated polydopamine microcapsule[J]. Chinese Journal of Chemical Engineering,2021,29:317-325.
[30] DU M,CHEN H,YE J,et al. One-pot synthesis of efficient carbonic anhydrase-zeolitic imidazolate framework-8 composite for enhancing CO2 absorption[J]. Journal of CO2 Utilization,2020,40:101211.
[31] JUN S H,YANG J,JEON H,et al. Stabilized and immobilized carbonic anhydrase on electrospun nanofibers for enzymatic CO2 conversion and utilization in expedited microalgal growth[J]. Environmental Science & Technology,2020,54(2):1223-1231.
[32] SHAO P,CHEN H,YING Q,et al. Structure-activity relationship of carbonic anhydrase enzyme immobilized on various silica-based mesoporous molecular sieves for CO2 absorption into a potassium carbonate solution[J]. Energy & Fuels,2020,34(2):2089-2096.
[33] MOON H,KIM S,JO B H,et al. Immobilization of genetically engineered whole-cell biocatalysts with periplasmic carbonic anhydrase in polyurethane foam for enzymatic CO2 capture and utilization[J]. Journal of CO2 Utilization,2020,39:101172.
[34] WEN H,ZHANG L,DU Y,et al. Bimetal based inorganic-car-bonic anhydrase hybrid hydrogel membrane for CO2 capture[J]. Journal of CO2 Utilization,2020,39:101171.
[35] JIAO M,HE J,SUN S,et al. Fast immobilization of human carbonic anhydrase II on Ni-Based metal-organic framework nanorods with high catalytic performance[J]. Catalysts,2020,10(4):401.
[36] EFFENDI S S W,CHIU C Y,CHANG Y K,et al. Crosslinked on novel nanofibers with thermophilic carbonic anhydrase for carbon dioxide sequestration[J]. InternationalJournal of Biological Macromolecules,2020,152:930-938.
[37] 王静. 碳酸酐酶用于二氧化碳捕集的研究进展 [J]. 化学工业与工程技术,2012(6):40-43.
WANG Jing. Research progress of carbon dioxide capture by using carbonic anhydrase[J]. Journal of Chemical Industry & Engineering,2012(6):40-43.
[38] BORHANI T N,WANG M. Role of solvents in CO2capture processes:The review of selection and design methods[J]. Renewable and Sustainable Energy Reviews,2019,114:109299.
[39] 刘彬. 碳酸酐酶催化醇胺溶液吸收CO2动力学及反应机理研究 [D].长沙:湖南大学,2017.
LIU Bin. Study on Mechanism and kinetics of CO2 absorption into alcohol amine catalyzed by carbonic anhydrase[D]. Changsha:Hunan University,2017.
[40] SILVERMAN D N,LINDSKOG S. The catalytic mechanism of carbonic anhydrase:Implications of a rate-limiting protolysis of water[J]. Accounts of Chemical Research,1988,21(1):30-36.
[41] GLADIS A,GUNDERSEN M T,NEERUP R,et al. CO2 mass transfer model for carbonic anhydrase-enhanced aqueous MDEA solutions[J]. Chemical Engineering Journal,2018,335:197-208.
[42] SHARMA T,SHARMA S,KAMYAB H,et al. Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases:A review[J]. Journal of Cleaner Production,2020,247:119138.
[43] LEIMBRINK M,SANDK MPER S,WARDHAUGH L,et al. Energy-efficient solvent regeneration in enzymatic reactive absorption for carbon dioxide capture[J]. Applied Energy,2017,208:263-276.
MPER S,WARDHAUGH L,et al. Energy-efficient solvent regeneration in enzymatic reactive absorption for carbon dioxide capture[J]. Applied Energy,2017,208:263-276.
[44] FERON P H M. Exploring the potential for improvement of the energy performance of coal fired power plants with post-combustion capture of carbon dioxide[J]. International Journal of Greenhouse Gas Control,2010,4(2):152-160.
[45] GLADIS A,GUNDERSEN M T,FOSBØL P L,et al. Influence of temperature and solvent concentration on the kinetics of the enzyme carbonic anhydrase in carbon capture technology[J]. Chemical Engineering Journal,2017,309:772-786.
[46] LEIMBRINK M,NIKOLEIT K G,SPITZER R,et al. Enzymatic reactive absorption of CO2 in MDEA by means of an innovative biocatalyst delivery system[J]. Chemical Engineering Journal,2018,334:1195-1205.
[47] GLADIS A,LOMHOLDT N F,FOSBØL P L,et al. Pilot scale absorption experiments with carbonic anhydrase-enhanced MDEA-Benchmarking with 30% MEA[J]. International Journal of Greenhouse Gas Control,2019,82:69-85.
[48] AI S,DANG B,LV B,et al. Absorption characteristics and kinetics of CO2 capture into N-methyldiethanolamine aqueous solution catalyzed by the immobilized carbonic anhydrase[J]. Biocatalysis and Biotransformation,2019,37(5):331-340.
[49] 刘彬,刘贺磊,梁志武. 碳酸酐酶催化N-甲基二乙醇胺吸收CO2的动力学研究[C]//第十八届中国科协年会——分8煤化工精细化发展论坛论文集. 西安:中国科学技术协会学会学术部,2016.
LIU Bin,LIU Helei,LIANG Zhiwu. Kinetic study of CO2 absorption into aqueous MDEA solution catalyzed by carbonic anhydrase[C]//The 18th Annual Meeting of China Association for Science and Technology-the proceedings of the Forum on refined Development of Coal Chemical Industry.Xi′an:Academic Department of the Society of China Association for Science and Technology,2016.
[50] 党博文. 固定化碳酸酐酶催化N-甲基二乙醇胺捕集CO2的研究 [D]. 厦门:华侨大学,2017.
DANG Bowen. Study on trapping CO2 with N-methyl diethanolamine by immobilized carbonic anhydrase[D]. Xiamen:Huaqiao university,2017.
[51] LEIMBRINK M,LIMBERG T,KUNZE A K,et al. Different strategies for accelerated CO2 absorption in packed columns by application of the biocatalyst carbonic anhydrase[J].Energy procedia,2017,114:781-794.
[52] QI G,LIU K,HOUSE A,et al. Laboratory to bench-scale evaluation of an integrated CO2 capture system using a thermostable carbonic anhydrase promoted K2CO3 solvent with low temperature vacuum stripping[J]. Applied Energy,2018,209:180-189.
[53] PHAN D T,BURNS R C,PUXTY G,et al. A study of bovine and human carbonic anhydrases as a model enzyme system for CO2 hydration in post combustion capture[J]. International Journal of Greenhouse Gas Control,2015,37:85-89.
[54] HU G,SMITH K H,NICHOLAS N J,et al. Enzymatic carbon dioxide capture using a thermally stable carbonic anhydrase as a promoter in potassium carbonate solvents[J]. Chemical Engineering Journal,2017,307:49-55.
[55] LI L,FU M,ZHAO Y,et al. Characterization of carbonic anhy-drase II from Chlorella vulgaris in bio-CO2 capture[J]. Environmental Science and Pollution Research,2012,19(9):4227-4232.
[56] BOND G M,STRINGER J,BRANDVOLD D K,et al. Develop-ment of integrated system for biomimetic CO2 sequestration using the enzyme carbonic anhydrase[J]. Energy & Fuels,2001,15(2):309-316.
[57] 李娟,张琳,孙莹,等.固定化碳酸酐酶催化吸收模拟烟气中CO2实验研究[J].化工进展,2017,36(9):3502-3507.
LI Juan,ZHANG Lin,SUN Ying,et al. Experimental studies on catalytic absorption of CO2 in simulated flue gas by immobilized carbonic anhydrase[J]. Chemical Industry and Engineering Progress,2017,36(9):3502-3507.
[58] FARIDI S,SATYANARAYANA T. Characteristics of recombinant α-carbonic anhydrase of polyextremophilic bacterium Bacillus halodurans TSLV1[J]. International journal of biological macromolecules,2016,89:659-668.
[59] RAMANAN R,KANNAN K,SIVANESAN S D,et al. Bio-sequestration of carbon dioxide using carbonic anhydrase enzyme purified from Citrobacter freundii[J]. World Journal of Microbiology and Biotechnology,2009,25(6):981-987.
[60] SUNDARAM S,THAKUR I S. Induction of calcite precipitation through heightened production of extracellular carbonic anhydrase by CO2 sequestering bacteria[J]. Bioresource Technology,2018,253:368-371.
[61] LI W,LIU L,CHEN W,et al. Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors[J]. Process Biochemistry,2010,45(6):1017-1021.
[62] CIZER Ö,RUIZ-AGUDO E,RODRIGUEZ-NAVARRO C. Kinetic effect of carbonic anhydrase enzyme on the carbonation reaction of lime mortar[J]. International Journal of Architectural Heritage,2018,12(5):779-789.
[63] ZHUANG D,YAN H,TUCKER M E,et al. Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2 concentrations:Further insights into biotic and abiotic calcite[J]. Chemical Geology,2018,500:64-87.
[64] ZHENG T,QIAN C. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase[J]. Process Biochemistry,2020,91:271-281.
[65] LI W,CHEN W S,ZHOU P P,et al. Influence of initial calcium ion concentration on the precipitation and crystal morphology of calcium carbonate induced by bacterial carbonic anhydrase[J]. Chemical Engineering Journal,2013,218:65-72.
[66] LI W,CHEN W S,ZHOU P P,et al. Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase[J]. Colloids and Surfaces B:Biointerfaces,2013,102:281-287.
[67] 袁亮. 微生物碳酸酐酶诱导CaCO3沉淀的影响因素及生成机理[J]. 生物技术通报,2020,36(8):79-86.
YUAN Liang. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase[J]. Biotechnology Bulletin,2020,36(8):79-86.