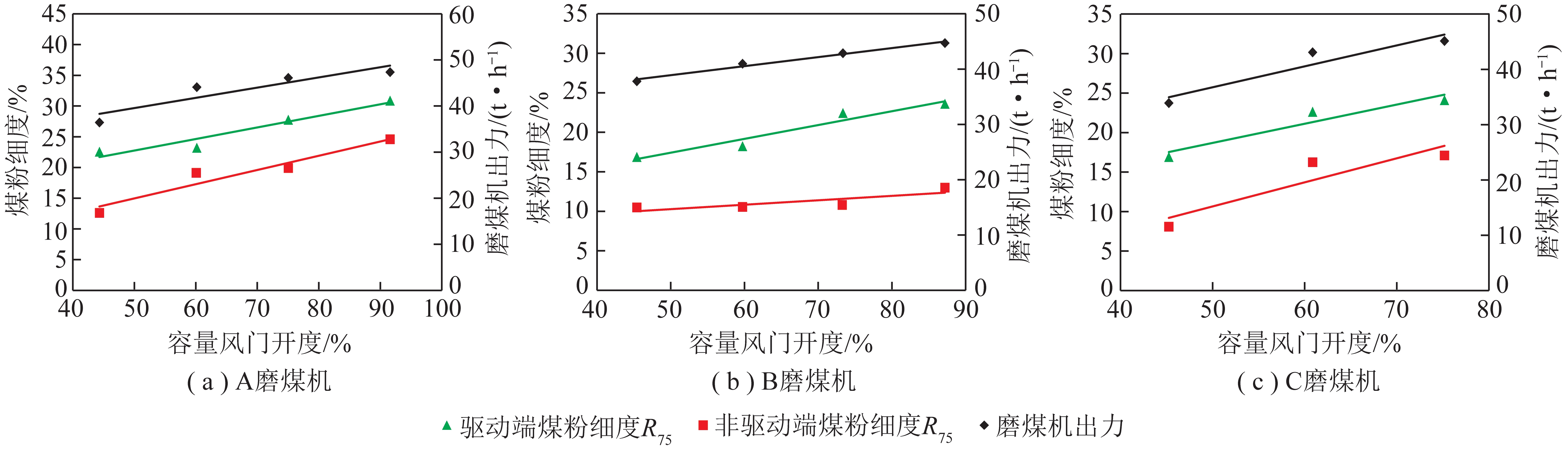
“双碳时代CH4资源化利用关键技术”专题
CH4-CO2重整镍基催化剂抗积碳性能研究进展
0 引 言
工业革命以来,大气中的CH4、CO2等温室气体排放量不断增加,温室效应愈发显著[1]。为应对全球气候变暖,履行《巴黎协定》,2020年9月22日,中国郑重向国际社会宣布力争在2030年前碳达峰、2060年前实现碳中和。2021年11月10日,经中美签订《中美关于在21世纪20年代强化气候行动的格拉斯哥联合宣言》[2],特别声明两国要强化CH4检测、减少CH4排放量。双碳减排是中国可持续发展和高质量发展的内在需要,也是我国对国际社会的重要承诺。
CH4为天然气的主要成分,为减少对有限石油和煤炭资源的依赖,以CH4为原料,可开发高效的CH4转化工艺及催化剂,生产高附加值的化学品和液体燃料等[3]。CH4具有高度对称的四面体结构,性质稳定,直接转化较为困难[4]。工业上以间接转化为主[4],即先通过重整将CH4转化成合成气,合成气进一步转化制备燃料和化学品等。CH4重整转化工艺主要有CH4蒸气重整(SRM)、CH4部分氧化(POM)以及CH4-CO2重整(DRM)等[5]。相比另外2种工艺,DRM反应消耗的原料气是温室气体的主要成分——CH4和CO2,且生成合成气的H2/CO物质的量比更接近1,可作为二甲醚合成、F-T合成液体碳氢化合物、氢甲酰化等反应的原料气[6]。不仅可以消耗、利用温室气体,同时也有利于缓解原油资源面临枯竭的危机。DRM反应中镍基催化剂因其价格低廉且活性与贵金属催化剂相近而被广泛研究,但其易因积碳失活,抗积碳性能有待提高[7]。笔者就镍基催化剂积碳问题从DRM反应热力学、DRM反应机理、催化剂积碳失活机理、催化剂组成及制备方法等进行了综述。
1 CH4-CO2重整热力学
DRM反应过程的副反应不可避免,主要涉及的反应有:
① CH4-CO2重整反应:

(1)
② CH4裂解反应:

(2)
③ CO化反应:

(3)
④ 逆水煤气变换反应:

(4)
由吉布斯焓变(ΔH)可知,除CO歧化反应(3)是放热反应外,其他反应均为吸热反应。主反应(1)为强吸热可逆反应,因此平衡向生成合成气方向移动的有利条件是高温低压。由于发生逆水煤汽变换反应(4)会消耗原料气中CO2产生CO,因此一般情况下CO2转化率高于CH4转化率,H2/CO物质的量比小于1。上述各反应温度极限见表1。
表1 CH4干重整反应过程主要反应的温度极限
Table 1 Temperature limits of main reactions in dry
reforming of methane

积碳主要来源于反应(2)和(3),反应温度557~700 ℃时最有可能产生积碳[8]。反应温度高于700 ℃时,不利于CO歧化反应(3)的进行。随温度继续升高,积碳将主要来源于CH4裂解反应(2)[9]。林小荣[10]模拟计算获得了CH4-CO2重整反应(0.1 MPa、CH4物质的量为1 kmol、CO2/CH4物质的量比为1,假定反应会产生积碳)热力学稳定时,各组分物质的量随温度变化如图1所示。反应温度不超过900 ℃时,H2/CO物质的量比较大,归因于CH4裂解(生成了部分H2)和CO歧化反应(消耗了CO);而温度超过900 ℃后,H2/CO物质的量比接近1,积碳生成明显受到抑制。

图1 CH4干重整反应热力学平衡组成[10]
Fig.1 Thermodynamic equilibrium composition
for dry reforming of methane[10]
2 CH4-CO2重整反应机理
DRM反应涉及的副反应较多,过程复杂,且由于催化剂体系内组分不同及反应操作条件等不确定因素,CH4-CO2反应至今没有明确统一的机理。
目前,普遍认为CH4在活性金属上活化,其依次脱氢裂解最终产生碳物种的过程为
CH4![]() CH4*
CH4*![]() CHx* (4-x)H*
CHx* (4-x)H*![]() C* 4H*。
C* 4H*。
(5)
EDRBEYLI等[11]和MARK等[12]提出的Eley-Rideal反应机理认为在以贵金属作为活性组分的催化反应中,CO2可以直接与CH4裂解过程中产生的碳物种反应生成CO,而不需要经过吸附过程:
CO2 C*![]() 2CO *。
2CO *。
(6)
WANG等[13]提出了CH4和CO2的活化途径,如图2所示,CH4-CO2重整包括CH4解离、CO2解离、C*氧化和CH*氧化途径。其中,CO2可直接解离为CO*和O*。在C*氧化途径中,CH4依次进行3次脱氢解离为CH*,CH*再解离形成C*,C*与O*形成CO*偶合;在CH*氧化途径中,CH4先进行3次脱氢解离生成CH*,CH*被O*氧化为CHO*,接着CHO*分解为CO*和H*。

图2 CH4和CO2活化途径[13]
Fig.2 Activation pathways of methane and carbon dioxide[13]
KROLL等[14]研究表明CH4和CO2分别在不同活性位点上活化(式(7)、(8)),其中, 为载体表面活性位。CO主要由CO2在载体表面解离产生的氧物种与催化剂上沉积的碳物种反应而生成。ZHANG等[15]研究同样表明CO2活化发生在催化剂载体上。
为载体表面活性位。CO主要由CO2在载体表面解离产生的氧物种与催化剂上沉积的碳物种反应而生成。ZHANG等[15]研究同样表明CO2活化发生在催化剂载体上。
CO2 
![]() CO2
CO2
![]() CO O
CO O ,
,
(7)
C* O
![]() CO *
CO *  。
。
(8)
ROSTRUPNIELSEN等[16]发现CO2在过渡金属上活化解离过程可发生在活性组分上:
CO2 *![]() CO O*,
CO O*,
(9)
CHx* O*![]() CO 0.5xH2 2*。
CO 0.5xH2 2*。
(10)
BRANDFORD等[17]研究发现CO2通过逆水煤气变换反应生成羟基基团,表面羟基基团与吸附的CHx中间体在活性中心与载体交界面处反应生成中间体CHxO,其分解产生CO。
TSIPOURIARI等[18]研究表明Ni/La2O3催化剂在DRM反应过程中,载体La2O3与CO2反应生成了La2O2CO3,La2O2CO3可分解产生CO或提供氧物种,使其与CH4裂解沉积在镍微晶上的碳物种反应生成CO。涉及过程如下:
CO2 La2O3![]() La2O2CO3,
La2O2CO3,
(11)
La2O2CO3 C*![]() La2O3 2CO *。
La2O3 2CO *。
(12)
ALDANA等[19]基于Ni-CeZr溶胶凝胶催化体系提出的反应机理,如图3所示。CO2在CeZr载体上被活化,生成碳酸盐物种,之后被氢化生成甲酸盐物种,最终生成甲氧基物种。这一反应机理也用于解释不同体系中的CH4-CO2重整反应途径[20-26]。

图3 Ni-CeZr催化剂上反应机理[19]
Fig.3 Reaction mechanism on Ni-CeZr catalyst[19]
尽管各体系中活化机理存在较大差异,但研究表明,CH4在金属表面上脱氢裂解。CO2在载体上的活化随载体酸碱性的不同活化路径有所差异,而对于惰性载体,CO2活化发生在活性金属上。
3 催化剂积碳失活机理分析
工业上要求催化剂具有高活性、抗积碳及较长的使用寿命[27],以防止频繁停车更换催化剂带来的损失。CH4-CO2重整镍基催化剂制备合成气反应过程中的主要问题是催化剂因积碳失活。

图4 Ni/CeO2催化剂上发生的积碳与消碳过程[28]
Fig.4 Carbon deposition and decarbonization
on Ni/CeO2 catalyst[28]
CH4-CO2催化重整过程中积碳主要来源于CH4裂解和CO歧化反应[9]。催化剂上积碳与消碳过程如图4所示。CH4分子在金属表面脱氢产生碳物种CHx(x=0~3)(CH4![]() C 2H2),未及时与CO2分子解离吸附产生的表面氧物种反应生成CO(C CO2
C 2H2),未及时与CO2分子解离吸附产生的表面氧物种反应生成CO(C CO2![]() CO)的碳物种可能会在金属表面发生深度脱氢和累积,形成积碳[28]。催化剂表面积碳依据活性不同可分为无定形碳和石墨碳,无定形碳在低于573 K时即可被氢气或含氧物种消除[29]。而石墨碳则需要在较高温度才能被气化消除,一般气化温度需高于923 K,且生成的惰性碳部分会堵塞催化剂孔道,降低催化剂孔隙率,部分会覆盖在催化剂表面,从而阻碍反应气体分子与活性组分的充分接触,是造成催化剂失活的主要原因[30]。
CO)的碳物种可能会在金属表面发生深度脱氢和累积,形成积碳[28]。催化剂表面积碳依据活性不同可分为无定形碳和石墨碳,无定形碳在低于573 K时即可被氢气或含氧物种消除[29]。而石墨碳则需要在较高温度才能被气化消除,一般气化温度需高于923 K,且生成的惰性碳部分会堵塞催化剂孔道,降低催化剂孔隙率,部分会覆盖在催化剂表面,从而阻碍反应气体分子与活性组分的充分接触,是造成催化剂失活的主要原因[30]。
对反应条件进行适当调变可以减少碳沉积。GADALLA等[31-32]在Ni/Al2O3催化剂上研究发现,增加原料气中CO2/CH4物质的量比可以抑制碳形成,且不同CO2/CH4物质的量比及操作压力,都有其对应的最佳操作温度范围,低于该温度范围会形成碳,而高于该温度范围会形成碳化镍。NEMATOLLAHI等[33]在各种压力下进行相同热力学模拟研究,发现随操作压力增加,CH4和CO2转化率及H2/CO物质的量比显著降低。但工业上要求降低反应温度的同时,CO2/CH4物质的量比要接近1。这就需要在设计催化剂时,保证较高反应活性,同时抑制积碳的生成[34]。
SACCO等[35]和ROSTRUP-NIELSEN等[36]探究了CH4干重整反应中发生碳沉积的条件。C—H—O相图(气相,101.325 Pa)如图5所示,可知CH4和CO2之间的线非常接近碳沉积阶段,因此在热力学上积碳很容易发生。在DRM反应过程中,惰性碳来源于CH4分解和CO歧化。前者是一个吸热过程,催化剂床层入口处积碳主要来源于此,出口处积碳来源于后者,后者为放热过程。在高温下,负载型金属催化剂易因烧结[37]或与载体发生不可逆反应而失活,如形成惰性尖晶石。但即使在高温不受热力学影响的情况下,该反应仍不可避免伴随着碳沉积[17,38]。因此,需开发一种热力学稳定的催化剂,以防止由于碳沉积或烧结而导致的催化剂失活[37-41]。

图5 C—H—O相图[35-36]
Fig.5 C—H—O phase diagram[35-36]
此外,将水蒸气或氧气加入反应原料进行混合重整也可减轻催化剂上的碳沉积[42]。尽管已经有许多研究通过上述途径减少积碳,但如何彻底解决镍基催化剂积碳问题仍是工业实施过程中的一个重大挑战。
4 CH4-CO2重整催化剂
镍基催化剂的积碳失活是阻碍工业应用的主要原因。催化剂的研究是解决反应积碳问题的核心。CH4-CO2重整反应基本在热力学积碳区内进行,因此在保证催化剂高催化活性的同时抑制积碳的形成尤为重要。如设计保持催化剂活性的同时使碳消除速率大于生成速率,或二者速率维持平衡,催化剂表面积碳现象得到有效抑制,从而获得一种高活性、长寿命的催化剂,以满足其规模化工业应用的要求[43]。为此,研究者分别从活性组分、载体、助剂及制备方法等方面开展了大量探索,获得了一系列有价值的研究结果。
4.1 活性组分
4.1.1 镍颗粒尺寸减小
CH4裂解反应为结构敏感反应[44-45],即相较于Ni(111)晶面此反应更易发生在Ni(100)和Ni(110)晶面上[44]。且在Ni(110)晶面上产生的碳比在Ni(100)上的碳更易扩散到本体中[45]。而相较于在较小金属颗粒上吸附的碳,在较大金属颗粒上吸附的碳更易发生扩散[46]。其他研究人员也发现了类似结论,当金属团簇尺寸大于临界尺寸时,催化剂较易产生积碳[47]。减小金属镍颗粒尺寸使其在载体上高度分散会破坏扩展碳结构的形成,增强载体与金属之间的相互作用力,增加载体上CO2解离形成的O原子氧化CH4解离形成的C原子概率,从而减少碳沉积[16]。
HU等[48]研究表明,镍颗粒尺寸越小,分散越均匀,催化剂表现出的抗积碳性能越好。WANG等[49]认为积碳含量、种类与位置不同,对于最终催化剂的失活有较大影响,其中与镍晶粒接触良好的一小部分积碳一般较易消除。XU等[50]制备了Ni/La2O3/γ-Al2O3和Ni/La2O3/α-Al2O3催化剂并发现,Ni颗粒尺寸小于15 nm时积碳明显减少(图6),使催化剂具有更高的催化活性和稳定性。 等[51]研究发现金属活性颗粒小于6 nm的催化剂表面具有优异的抗积碳能力。LIU等[52]发现镍颗粒小于5 nm时,对积碳有明显的抑制作用。以上研究表明,减小镍颗粒尺寸可以有效改善镍基催化剂的积碳问题。
等[51]研究发现金属活性颗粒小于6 nm的催化剂表面具有优异的抗积碳能力。LIU等[52]发现镍颗粒小于5 nm时,对积碳有明显的抑制作用。以上研究表明,减小镍颗粒尺寸可以有效改善镍基催化剂的积碳问题。

图6 反应后催化剂TEM图片[50]
Fig.6 TEM images of catalyst after reaction[50]
4.1.2 双金属镍基催化剂
与单一金属镍基催化剂相比,双金属镍基催化剂RhNi[53-54]、PtNi[55]、PdNi[56]、CoNi[57]和CuNi[58]等在CO2与CH4重整反应中表现出更好的活性和抗积碳性能。LUCRÉDIO等[59]研究了RhNi/Al2O3催化剂,发现添加贵金属Rh可通过氢溢流促进Ni的还原,使催化剂具有较高的活性和稳定性。GARCI -DIÉGUEZ等[60]制备了NiPt双金属催化剂用于DRM反应,与Ni催化剂相比,NiPt双金属催化剂形成了NiPt合金,具有更高的活性和较低的碳沉积。笔者课题组前期[61]通过浸渍法制备了介孔γ-Al2O3(MA)负载的Ni、Pd和NiPd催化剂,如图7所示。活性组分颗粒非常细小并且均匀分散在MA载体上(图7(a)),其中PdNi颗粒在透射电子显微镜下可以观察到有序的金属晶格条纹,晶格间距为0.220 nm(图7(a)),其介于纯Pd(111)晶面(0.225 nm)和Ni(111)晶面(0.203 nm)的相应晶格间距之间,表明在Pd-Ni/MA表面形成Pd-Ni合金。与单独负载镍或钯的催化剂相比,双金属NiPd/MA催化剂的活性更高(图7(b)、(c))。
-DIÉGUEZ等[60]制备了NiPt双金属催化剂用于DRM反应,与Ni催化剂相比,NiPt双金属催化剂形成了NiPt合金,具有更高的活性和较低的碳沉积。笔者课题组前期[61]通过浸渍法制备了介孔γ-Al2O3(MA)负载的Ni、Pd和NiPd催化剂,如图7所示。活性组分颗粒非常细小并且均匀分散在MA载体上(图7(a)),其中PdNi颗粒在透射电子显微镜下可以观察到有序的金属晶格条纹,晶格间距为0.220 nm(图7(a)),其介于纯Pd(111)晶面(0.225 nm)和Ni(111)晶面(0.203 nm)的相应晶格间距之间,表明在Pd-Ni/MA表面形成Pd-Ni合金。与单独负载镍或钯的催化剂相比,双金属NiPd/MA催化剂的活性更高(图7(b)、(c))。

图7 NiPd催化剂TEM图片和催化活性[61]
Fig.7 TEM images of NiPd catalyst and catalytic activity[61]
Fe和Ni均为铁系元素,在元素周期表中处于第4周期第Ⅷ族,元素性质相近,在一定比例下可形成合金,从而使催化剂具有良好的催化性能[57,62]。KIM等[63]研究表明,单独负载Ni或Fe的催化剂分别存在快速失活和低转化率的问题,而双金属NiFe催化剂在DRM反应中表现出良好的活性和稳定性。通过表征手段阐明了Fe在NiFe合金中的促进作用,如图8所示。CH4在活性金属Ni上裂解生成H2和碳。部分Fe与CO2反应生成为FeO并脱离合金,碳可与FeO反应,碳被氧化生成CO,FeO被还原为Fe并恢复为原始的NiFe合金。体系中这一消碳反应循环,有利于减少催化剂表面的碳沉积。

图8 NiFe合金作用机理[63]
Fig.8 Action mechanism of NiFe alloys[63]
通常在镍基催化剂中引入第二活性组分Cu或Co有助于提高催化活性和抗积碳性能[64]。WU等[58]通过水热合成Cu-Ni/SiO2催化剂,其中Cu和Ni形成合金,抑制了金属烧结和积碳的形成。在NiCo合金中,Co的强亲氧性[65]可以提高催化剂表面O*的浓度,有助于消除积碳[66]。SAN-JOSÉ-ALONSO等[57]用γ-Al2O3作为载体,分别制备了Ni、Co以及Ni-Co双金属催化剂用于CH4干重整反应,Ni-Co双金属催化剂表现出较单一活性组分更好的活性和稳定性。ZHANG等[67]制备了Ni-Me-Al-Mg-O(Me=Co、Fe、Cu或Mn)双金属催化剂和Ni(Co)单金属催化剂,催化活性顺序为NiCo>NiMn>NiFe>NiCu,NiCo>Co>Ni,镍钴双金属催化剂的优异性能来自双金属协同效应、良好的金属分散性以及较强的相互作用。总之,双金属催化剂可在一定程度上形成合金,合金中的双金属在活化CH4及消碳过程中起协同作用,从而提高了双金属镍基催化剂对DRM反应催化活性和抗积碳能力。
4.2 载体
载体通过为活性组分提供物理支撑或与其发生相互作用来影响催化剂的结构和性能[68]。此外,载体的酸碱性、氧化还原性、孔结构等均会影响其催化性能[69]。DRM反应一般需在较高温度下进行,这对催化剂载体的热稳定性提出了较高要求[69]。通常选择Al2O3、SiO2、MgO、ZrO2、La2O3、CeO2以及Al2O3-MgO、Al2O3-ZrO2、MgAl2O4等复合金属氧化物作为催化剂载体[70]。
4.2.1 金属-载体相互作用
在一定程度上,通过调节金属-载体相互作用(Metal-Support Interactions,MSI)可以改变催化剂表面活性组分的晶粒尺寸、分散性、还原性,进而影响催化剂的催化活性和抗积碳性能[71-72]。DRM反应中,强的金属-载体相互作用(Strong Metal-Support Interactions,SMSI),更有利于得到高度分散且颗粒尺寸较小的镍颗粒[73],但过强的MSI可能需要较高的还原温度还原出金属镍,因而导致金属烧结。CHEN等[74]通过探究Ni/Al2O3催化剂焙烧温度对MSI的影响,表明焙烧温度越高,Ni-Al2O3之间的相互作用力就越强,焙烧温度超过973 K,体系中可能会产生NiAl2O4。将不同焙烧温度制备的Ni/Al2O3催化剂分别在反应温度873和1 073 K进行活性评价表明,873 K时,焙烧温度越高的催化剂活性越低。而1 073 K高温反应,催化剂活性差异较小,但催化剂焙烧温度的升高对抑制积碳有明显作用。ZHANG等[75]采用柠檬酸溶胶凝胶法合成了一系列具有NiAl2O4尖晶石相的NixAl1O2-δ介孔催化剂应用于CH4干重整反应,研究表明,Ni含量较低的NixAl1O2-δ介孔催化剂中,NiAl2O4尖晶石在高温部分还原成晶粒尺寸较小且高度分散的活性Ni微晶,从而在动力学上可以有效抑制碳的生成。HORV TH等[76]选择尖晶石结构MgAl2O4作为Ni基催化剂载体,通过溶胶凝胶法制得AuNi/MgAl2O4催化剂,研究发现,SMSI有助于提高金属分散性,而载体本身的碱性促进了CO2吸附,有利于消除积碳。WANG等[77]将Co通过共沉淀添加到Ni/MgAl2O4中,其中Ni和Co的相互作用以及NiCo与MgAl2O4间的相互作用减缓了Ni的还原并生成了小且分散均匀的Ni颗粒,从而提高催化DRM反应活性。Ni/MgO中NiO-MgO固溶体的形成导致一部分NiO溶解在MgO相中,还有少部分NiO留在载体表面。溶解在MgO相中的活性中心Ni较难被还原出来,当载体表面少量Ni被烧结时,溶解在MgO中的NiO可被反应中产生的氢还原成Ni从而得到补偿,进而提升了催化剂的稳定性和抗积碳性能[78]。RUCKENSTEIN等[79]采用浸渍法制备了Ni/TiO2催化剂,发现Ni与TiO2之间存在强的相互作用,导致体系自由能降低,TiOx在一定程度上可促进碳消除,但还原过程中TiOx分子会在表面迁移覆盖Ni活性位点。
TH等[76]选择尖晶石结构MgAl2O4作为Ni基催化剂载体,通过溶胶凝胶法制得AuNi/MgAl2O4催化剂,研究发现,SMSI有助于提高金属分散性,而载体本身的碱性促进了CO2吸附,有利于消除积碳。WANG等[77]将Co通过共沉淀添加到Ni/MgAl2O4中,其中Ni和Co的相互作用以及NiCo与MgAl2O4间的相互作用减缓了Ni的还原并生成了小且分散均匀的Ni颗粒,从而提高催化DRM反应活性。Ni/MgO中NiO-MgO固溶体的形成导致一部分NiO溶解在MgO相中,还有少部分NiO留在载体表面。溶解在MgO相中的活性中心Ni较难被还原出来,当载体表面少量Ni被烧结时,溶解在MgO中的NiO可被反应中产生的氢还原成Ni从而得到补偿,进而提升了催化剂的稳定性和抗积碳性能[78]。RUCKENSTEIN等[79]采用浸渍法制备了Ni/TiO2催化剂,发现Ni与TiO2之间存在强的相互作用,导致体系自由能降低,TiOx在一定程度上可促进碳消除,但还原过程中TiOx分子会在表面迁移覆盖Ni活性位点。
4.2.2 载体的酸碱性
通过调节催化剂载体的酸碱性可以改变CO2的吸附能力进而影响重整催化剂的催化性能。大多数研究者认为DRM机理遵循双功能途径,CH4在活性金属上被活化,CO2在酸性载体上与表面羟基形成甲酸盐而活化,在碱性载体上,CO2通过形成碳酸盐而活化[80]。而对于相对惰性材料(如SiO2)作为载体的催化剂则遵循单功能途径机理,即CH4和CO2活化均发生在金属上。在惰性载体上,CH4裂解脱氢形成碳物种会吸附在金属表面并累积,最终限制后续的CO2活化和CO2参与的消碳反应,导致催化剂易失活[80-81]。与酸性载体(Al2O3)或碱性载体(La2O3)制备的催化剂相比,惰性载体制备的催化剂具有相对较弱的金属-载体相互作用和较低的活性和稳定性[82-84],但对于双金属催化剂,采用惰性载体可以提供更好的金属-金属相互作用[85]。
DAS等[86]用酸性/碱性SiO2和Al2O3为载体制备Ni基催化剂,探究了载体酸碱性质对催化剂活性和失活的影响。研究表明,载体上过多的酸性位点会促使CH4裂解脱氢产生碳物种,导致催化剂形成积碳而失活。过多的碱性位点会造成金属氧化而发生Boudouard反应,从而引起催化剂失活。催化剂载体上均匀分布的酸性和碱性位点,使CH4裂解反应的碳沉积速率与碳被表面氧氧化的速率相当,且催化剂中SMSI与较小的镍颗粒有助于催化剂获得较高的活性。催化剂载体上不规则分布着酸性和碱性位点,会促进逆水煤气变换反应的发生和活性金属被表面吸附的氧氧化,沉积在活性位点上的积碳不易氧化,进而导致催化剂快速失活。
因此设计载体产生适度的酸碱性,可以尽可能降低干重整催化剂的失活。如采用La2O3作为载体,反应过程中形成的碳酸氧镧可以促进低能垒CO2解离、提供表面氧原子以有效消除甲烷C—H键活化过程中形成的表面C原子,从而减少积碳[82,87]。JAFARBEGLOO等[88]以碱性氧化物MgO为载体,采用一步法制备了镍基催化剂,研究表明具有碱性表面的镍镁固溶体的产生有利于抑制积碳的沉积。WANG等[89]分别以γ-Al2O3、SiO2、La2O3、MgO、TiO2等为载体制备镍基催化剂用于CH4干重整反应性能研究,研究表明,载体La2O3和MgO具备较强的碱性,有利于CO2吸附和活化而呈现出较高的反应活性和稳定性。JANG等[90]制备了Ni-Ce0.8Zr0.2O2与Ni-MgO-Ce0.8Zr0.2O2催化剂,并在800 ℃进行了DRM反应活性测试,结果表明,Ni-MgO-Ce0.8Zr0.2O2催化剂具有更高的活性,在200 h内CH4转化率均高于95%,其优异性能归因于MgO碱性对CO2的强吸附能力以及Ni和MgO之间较强的相互作用。
4.2.3 载体的氧化还原性
研究人员不断探索具有特殊氧化还原性质和超常储氧能力(OSC)的载体应用于CH4干重整反应,致力于最大限度利用氧空位促进CO2活化和解离,通过将表面碳氧化为CO来减少由于碳沉积而导致的催化剂失活[91-92]。具有氧化还原性质的载体可以直接与含碳反应物或中间体反应产生CO和H2,减少了碳的形成和沉积,从而改善了催化剂稳定性[93]。而具有高储氧能力的金属氧化物载体被高温还原时可以暴露出更多的氧晶格缺陷。CeO2因其氧化还原性能和高储氧能力被广泛研究,通过Ce3 和Ce4 之间的转变,释放晶格氧产生氧空位,加速了氧的迁移,促进了CO2吸附和活化,从而减少了表面的碳沉积[72]。通过密度泛函理论(DFT)计算表明Ni活化能为0.9 eV,而Ni-CeO2-x的活化能仅为0.15 eV[94]。较低的活化能可以提高反应速率和能量效率。催化剂上CH4和CO2的主要化学反应路径如图9所示,其中r为反应速率。CH4-CO2重整反应的决速步骤为CH4分子中C—H的活化[85]。当CeO2作为Ni基催化剂的载体,Ni与CeO2间会形成较强的相互作用,有利于CH4在DRM反应中的解离[95-96]。因此,采用具有氧化还原性和OSC载体制备DRM反应催化剂有望彻底解决镍基催化剂积碳问题。

图9 催化剂上CH4和CO2的主要化学反应路径[92]
Fig.9 Main chemical reaction paths of methane
and carbon dioxide on catalyst[92]
4.2.4 载体的孔结构
载体介孔结构的限域效应使镍颗粒尽可能存在于催化剂孔道中,且分散度较高,有利于形成较强的金属-载体相互作用,从而抑制Ni纳米粒子(NPs)的烧结,减少积碳的形成[97]。研究人员在Ni-SBA-15、Ni-KIT-6和Ni-meso-Al2O3等有序介孔催化剂上进行研究,发现介孔结构的限域效应不仅提高了活性位点的结构稳定性,阻碍了金属烧结,还抑制了积碳长大[97-99]。ZHANG等[100]以乙二醇(EG)为溶剂,采用浸渍法将Ni-EG配合物利用毛细作用进入SBA-15有序孔道中,制备了Ni/SBA-15催化剂,从而阻碍了Ni在干燥和煅烧过程中的迁移。ZHANG等[101]制备了CeO2掺杂的Ni/SBA-16催化剂,基于SBA-16中介孔的限域效应、Ni-CeO2的强相互作用以及CeO2对活性颗粒及载体骨架结构稳定性的作用,该催化剂在DRM反应中显示出良好的稳定性。
与此同时,催化剂的双孔结构也因其良好的抗积碳性能引起广泛关注。研究较多的为介孔和大孔,介孔可提供较大的比表面积,为金属活性中心提供锚固点,大孔有利于气体分子的快速传质[102]。笔者课题组[102]采用一步法制备了具有大-中孔结构的γ-Al2O3载体(MM-A),与介孔催化剂相比,反应后积碳量明显减少(图10)。表明在介孔结构中引入大孔,结合了介孔大的比表面积以及大孔提供的快速传质通道,可明显减少催化剂上的积碳。

图10 大-中孔结构催化剂TEM图片和催化性能[102]
Fig.10 TEM images and catalytic performance of macroporous-mesoporous catalysts[102]
此外,催化剂的分层设计包括核壳、蛋黄壳、嵌入和中空结构等,在空间上阻碍了Ni的迁移并防止团聚,有利于提高催化剂的稳定性并减少积碳[72,103]。
4.3 助剂
添加助剂可以通过改善催化剂表面酸碱性、增大活性组分的分散程度、控制活性金属与载体的相互作用以及调节金属原子的电子密度等进而影响CH4、CO2分子的解离性能[104]。DRM反应催化剂中一般选用碱金属、碱土金属以及稀土金属氧化物作为助剂[65]。
许峥等[105]探究了添加助剂K2O、Li2O、MgO、La2O3对Ni/γ-Al2O3催化剂反应活性及抗积碳性能的影响,发现添加助剂的催化剂都表现出一定的抗积碳性能,但MgO、La2O3的抗积碳性能比其他助剂效果更显著,这与添加助剂本身碱性及其对活性组分分散度的提高有关。ALIPOUR等[106]采用浸渍法将碱性助剂MgO掺杂到Al2O3载体上,增强了镍基催化剂碱性,不仅抑制了碳的形成和沉积,且提高了催化活性。DIAS等[107]制备了不同含量CaO的Ni/CaO/γ-Al2O3催化剂,通过研究发现添加CaO的催化剂表面碱性增强,使催化剂表面吸附CO2的能力有所提升,进而提高了反应活性。Ca作为助剂,有效增强了Ni和Al2O3之间的相互作用,从而促进了Ni的分散并阻碍了金属烧结[108]。而CeO2作为助剂具有较强的给电子能力,可以增加催化剂表面金属镍的d电子密度,有利于抑制CH4分子裂解而促进CO2分子解离,进而增强催化剂抗积碳能力[109]。
4.4 制备方法
不同制备方法会使催化剂活性金属颗粒尺寸、分散度以及催化剂形貌等存在较大差异,进而影响其催化性能和抗积碳能力。近年来,通过改进传统制备方法和不断研制新型制备技术,为解决催化剂积碳失活问题提供了新途径。
DRM反应传统的制备方法有浸渍法、共沉淀法、溶胶凝胶法等。SUN等[110]分别采用浸渍法和共沉淀法制备了Ni-CaO-ZrO2催化剂应用于CH4-CO2重整反应,结果表明与浸渍法制备的催化剂相比,采用共沉淀法制备的催化剂中SMSI使Ni和Zr颗粒尺寸更小,分散更均匀,有利于减轻金属烧结,使其具有更高的稳定性和抗积碳性能。AGHAMOHAMMADI等[111]采用溶胶凝胶法和浸渍法制备了Ni/Al2O3-CeO2催化剂,与浸渍法相比,溶胶凝胶法制备的催化剂中活性相分散度更高、粒径更小,催化活性更好。
基于传统制备方法提升抗积碳性能的局限性,改善传统制备方法以及研究新型制备技术成为热点。YANG等[112]基于传统浸渍法进行改进,采用新颖的P123辅助浸渍法制备了Ni/SBA-15(1/X,X=0、5、50、100、500、750)催化剂,并将其用于DRM反应,结果表明与不添加P123相比,P123/Ni物质的量比为1/500、1/100及1/50时,催化剂Ni分散性明显提高,催化剂活性较高,在750 ℃反应50 h表现出良好的稳定性及抗积碳性能。XU等[113]采用蒸发诱导自组装(EISA)策略制备了NiO-MgO-Al2O3催化剂,其中NiO和MgO在介孔骨架中高度分散,镍和载体之间形成了较强的相互作用,在一定程度上避免了金属颗粒的团聚,催化剂在700 ℃反应100 h后未出现失活现象,表现出较高的催化活性和长期稳定性。
近年来,研究人员致力于开发一种新颖且有前景的催化剂,并克服过渡金属催化剂抗积碳性能差的问题,扩大了催化剂制备材料的选择范围,而非局限于传统负载型金属氧化物催化剂。其中,二维六方氮化硼(h-BN)纳米片[26,114-115](机理如图11所示)、低成本天然黏土[116]和金属有机骨架(MOFs)[117]等新材料在抗烧结和减少金属团聚等方面具有优异表现。

图11 Ni/BN@mSiO2催化剂上DRM反应机理[26]
Fig.11 Mechanism of the DRM reaction
over the Ni/BN@mSiO2 catalyst[26]
MA等[118]采用湿化学法制备了在碳纳米管内外选择性负载镍纳米粒子的催化剂,并对其催化性能进行研究,如图12所示(W为催化剂质量,F为进料气体流速)。研究表明,由于碳纳米管的限制作用,负载在碳纳米管内部的NiO更易被还原,活性和稳定性更高。当活性金属掺杂惰性材料时,金属与载体以电荷转移、表面缺陷锚定、金属-载体界面和表面纳米结构重构的形式相互作用,对催化剂的氧化还原性能、金属分散度等性能产生影响,为设计新型催化剂以实现优异的活性和抗积碳性能提供了思路。

图12 镍纳米粒子催化剂选择性负载在碳
纳米管内部或外部用于CH4干重整反应[118]
Fig.12 Nickel nanoparticle catalysts were selectively
supported inside or outside carbon nanotubes for
dry reforming of methane[118]
此外,等离子体辅助[117,119]、太阳能驱动[120]、化学循环重组[121]、无机膜耦合[122-123]等新技术也广泛应用于DRM反应研究,有利于提高能源效率。
综上所述,通过改善活性组分、载体、助剂、制备方法等,可以显著提高催化剂活性和抗积碳性能。
5 结语与展望
1)DRM反应能有效利用2种温室气体,并将其转化为可用于化学品生产的合成气,具有良好的工业价值和工业应用前景。近年来,科研工作者致力于为CH4-CO2重整反应研发活性高、抗积碳性能强的催化剂。
2)设计催化剂产生强的金属-载体相互作用,合成小且高度分散的镍颗粒或利用双金属的协同作用,可以有效提高反应活性并减少碳沉积;添加碱性助剂、使用碱性或高储氧能力的载体,可以促进CO2的吸附与活化,从而加快碳的消除速率,进而提高催化剂抗积碳能力。
3)载体介孔结构的限域效应也可以抑制镍颗粒的长大和烧结。采用双孔、核壳、蛋黄壳、嵌入和中空结构等特殊结构,均有利于提高催化剂的稳定性并减少积碳。
4)改进传统制备方法或采用新型材料和制备技术也为CH4-CO2重整反应催化剂的制备提供了新思路。等离子体辅助、太阳能驱动、化学循环重组、无机膜耦合等新型制备技术将有利于提高能源效率并改善催化剂高温烧结和积碳失活。
5)反应过程中不同反应驱动方法是未来催化反应的方向,如光热协同催化以及光或热在磁场作用下对催化剂结构及反应过程机理的影响将成为未来研究方向。
[1] KERR R A. Global warming is changing the world [J]. Science,2007,316:188-190.
[2] 张博,李蕙竹,仲冰,等. 中国甲烷控排面临的形势、问题与对策 [J]. 中国矿业,2022,31(2):1-10.
ZHANG Bo,LI Huizhu,ZHONG Bing,et al. The situation,problems and countermeasures for the controls of China′s methane emissions[J]. China Mining Magazine,2022,31(2):1-10.
[3] HORN R,SCHLÖGL R. Methane activation by heterogeneous catalysis [J],Catalysis Letters,2015,145(1):23-39.
[4] 苏永庆,王萍,任年军,等. 甲烷直接转化的研究现状与展望[J]. 云南化工,2009,36(4):1-6.
SU Yongqing,WANG Ping,REN Nianjun,et al. Research status and prospect of methane direct conversion [J]. Yunnan Chemical Industry,2009,36(4):1-6.
[5] 周则龄,张萌,张俊峰,等. 钙钛矿型氧化物负载Ni催化剂上甲烷二氧化碳重整反应研究[J]. 燃料化学学报,2020,48(7):833-841.
ZHOU Zeling,ZHANG Meng,ZHANG Junfeng,et al. Methane reforming with carbon dioxide over the perovskite supported Ni catalysts [J]. Journal of Fuel Chemistry and Technology,2020,48(7):833-841.
[6] 沈朝峰,郝树宏,黄伟,等. Mo对Ni-Mg-Al水滑石催化CH4-CO2重整反应的影响 [J]. 太原理工大学学报,2014,45(2):239-243.
SHEN Chaofeng,HAO Shuhong,HUANG Wei,et al. Effect of different Mo content on CH4 reforming of CO2 over Ni-Mg-Al hydrotalcite catalyst [J]. Journal of Taiyuan University of Technology,2014,45(2):239-243.
[7] 孙楠楠,闻霞,王峰,等. Ni含量对介孔Ni-CaO-ZrO2催化剂上CH4-CO2重整反应的影响研究 [J] .精细化工,2010,27(10):1004-1008.
SUN Nannan,WEN Xia,WANG Feng,et al. Influence of Ni content on catalytic performance of Ni-CaO-ZrO2 catalysts in CH4-CO2 reforming [J]. Fine Chemicals,2010,27(10):1004-1008.
[8] 冉东,申威峰. 天然气制甲醇结合蒸汽转化(SMR)和二氧化碳重整(DMR)串并联新补碳工艺探究 [J]. 当代化工研究,2019(11):1-7.
RAN Dong,SHEN Weifeng. Study on natural gas based methanol production process by the combined process of steam and dry methane reforming[J]. Modern Chemical Research,2019(11):1-7.
[9] 刘继伟. 新型掺硅介孔碳负载镍基催化剂制备及其催化甲烷二氧化碳重整制合成气性能研究 [D]. 太原:太原理工大学,2019.
[10] 林小荣. 高效双孔镍基催化剂的构建及在甲烷二氧化碳重整反应中的催化性能研究 [D]. 广州:华南理工大学,2018.
[11] ERDOHELYI A,CSERENYI J,SOLYMOSI F. Activation of CH4 and its reaction with CO2 over supported Rh catalysts [J]. Journal of Catalysis,1993,141(1):287-299.
[12] MARK M F,MAIER W F. CO2-reforming of methane on supported Rh and Ir catalysts [J]. Journal of Catalysis,1996,164(1):122-130.
[13] WANG Z,CAO X M,ZHU J,et al. Activity and coke formation of nickel and nickel carbide in dry reforming:A deactivation scheme from density functional theory[J]. Journal of Catalysis,2014,311:469-480.
[14] KROLL V C H,SWAAN H M,LACOMBE S,et al. Methane reforming reaction with carbon dioxide over Ni/SiO2 catalyst:A mechanistic study [J]. Journal of Catalysis,1996,164(2):387-398.
[15] ZHANG J G,WANG H,DALAI A K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH4 [J]. Applied Catalysis A:General,2008,339(2):121-129.
[16] ROSTRUP-NIELSEN J R,HANSEN J H B. CO2-reforming of methane over transition metals [J]. Journal of Catalysis,1993,144(1):38-49.
[17] BRADFORD M C J,VANNICE M A. Catalytic reforming of meth-ane with carbon dioxide over nickel catalysts II. Reaction kinetics [J]. Applied Catalysis A:General,1996,142(1):97-122.
[18] TSIPOUDARI V A,VERYKIOS X E. Carbon and oxygen reacti-on pathways of CO2 reforming of methane over Ni/La2O3 and Al2O3 catalysts studied by isotopic tracing techniques [J]. Journal of Catalysis,1999,187(1):85-94.
[19] ALDANA P A U,OCAMPO F,KOBL K,et al. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia:Reaction mechanism by operando IR spectroscopy [J]. Catalysis Today,2013,215:201-207.
[20] PAN Q S,PENG J X,SUN T J,et al. Insight into the reaction route of CO2 methanation:Promotion effect of medium basic sites [J]. Catalysis Communications,2014,45:74-78.
[21] ZHANG S H,SHI C,CHEN B B,et al. Catalytic role of β-Mo2C in DRM catalysts that contain Ni and Mo [J]. Catalysis Today,2015,258:676-683.
[22] LI X Y,LI D,TIAN H,et al. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles [J]. Applied Catalysis B:Environmental,2017,202:683-694.
[23] QIAN L P,MA Z,REN Y,et al. Investigation of La promotion mechanism on Ni/SBA-15 catalysts in CH4 reforming with CO2 [J]. Fuel,2014,122:47-53.
[24] BACHILLER-BAEZA B,MATEOS-PEDRERO C,SORIA M A,et al. Transient studies of low-temperature dry reforming of methane over Ni-CaO/ZrO2-La2O3 [J]. Applied Catalysis B:Environmental,2013,129:450-459.
[25] STEIB M,LOU Y,JENTYS A,et al. Enhanced activity in meth-ry reforming by CO2 induced metal-oxide interface restructuring of Ni/ZrO2 [J]. ChemCatChem,2017,9(20):3809-3813.
[26] CAO Y,LU M,FANG J H,et al. Hexagonal boron nitride supported mesoSiO2-confined Ni catalysts for dry reforming of methane [J]. Chemical Communication,2017,53(54):7549-7552.
[27] 刘宏. 碳一化学:甲烷二氧化碳重整制合成气及甲醇转化制烯烃研究 [D]. 南京:南京大学,2013.
[28] LIANG T Y,LIN C Y,CHOU F C,et al. Gas-phase synthesis of Ni-CeOx hybrid nanoparticles and their synergistic catalysis for simultaneous reforming of methane and carbon dioxide to syngas [J]. The Journal of Physical Chemistry C,2018,122(22):11789-11798.
[29] 胡贤辉. 合成气制天然气镍基催化剂的研究 [D]. 上海:华东理工大学,2011.
[30] 任琳. 镍催化剂用于双气头多联产系统重整反应单元研究[D]. 太原:太原理工大学,2010.
[31] GADALLA A M,SOMMER M E. Carbon dioxide reforming of me-thane on nickel catalysts [J]. Chemical Engineering Science,1989,44:2825-2829.
[32] GADALLA A M,BOWER B. The role of catalyst support on the activity of nickel for reforming methane with CO2 [J]. Chemical Engineering Science,1988,43:3049-3062.
[33] NEMATOLLAHI B,REZAEI M,LAY E N,et al. Thermodynamic analysis of combined reforming process using Gibbs energy minimization method:In view of solid carbon formation [J]. Journal of Natural Gas Chemistry,2012,21(6):694-702.
[34] 张轲. 低Ni载量六铝酸盐催化剂上甲烷和二氧化碳重整制合成气反应研究 [D]. 长春:吉林大学,2009.
[35] SACCO J R A,GEURTS F,JABLONSKI G A,et al. Carbon deposition and filament growth on Fe,Co,and Ni foils using CH4-H2-H2O-CO-CO2 gas mixtures [J]. Journal of Catalysis,1989,119(2):322-341.
[36] ROSTRUP-NIELSEN J,TRIMM D L. Mechanisms of carbon formation on nickel-containing catalysts[J]. Journal of Catalysis,1977,48(1/3):155-165.
[37] HOU Z Y,CHEN P,FANG H L,et al. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-)promoted Ni catalysts [J]. International Journal of Hydrogen Energy,2006,31(5):555-561.
[38] GALLEGO G S,BATIOT-DUPEVRAT C,BARRAULT J,et al. Dry reforming of methane over LaNi1-yByO3±δ (B=Mg,Co) perovskites used as catalyst precursor [J]. Applied Catalysis A:General,2008,334(1/2):251-258.
[39] ZHANG Z L,VERYKIOS X E. Mechanistic aspects of carbon dioxide reforming of methane to synthesis gas over Ni catalysts [J]. Catalysis Letters,1996,38:175-179.
[40] GARC A-DIÉGUEZ M,PIETA I S,HERRERA M C,et al. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3 [J]. Catalysis Today,2010,149:380-387.
A-DIÉGUEZ M,PIETA I S,HERRERA M C,et al. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3 [J]. Catalysis Today,2010,149:380-387.
[41] LUO J Z,YU Z L,Ng C F,et al. CO2/CH4 Reforming over Ni-La2O3/5A:An investigation on carbon deposition and reaction steps[J]. Journal of Catalysis,2000,194(2):198-210.
[42] 邱业君. 甲烷催化转化制合成气镍基催化剂的研究 [D]. 天津:天津大学,2003.
[43] 詹宜秋. 镍基催化剂催化重整二氧化碳制备合成气研究 [D]. 武汉:武汉科技大学,2018.
[44] SCHOUTEN F C,GIJZEMAN O L J,BOOTSMA G A. Reaction of methane with nickel single crystal surfaces and the stability of surface nickel carbides [J]. Bulletin des Sociétés Chimiques Belges,1979,88(7/8):541-547.
[45] BARTHOLOMEW C H. Carbon deposition in steam reforming and methanation [J]. Catalysis Reviews Science and Engineering,1982,24(1):67-112.
[46] EIZENBERG M,BLAKELY J M. Carbon monolayer phase condensation on Ni (111) [J]. Surface Science,1979,82(1):228-236.
[47] AKBARI E,ALAVI S M,REZAEI M. CeO2 promoted Ni-MgO-Al2O3 nanocatalysts for carbon dioxide reforming of methane [J]. Journal of CO2 Utilization,2018,24:128-138.
[48] HU Y H,RUCKENSTEIN E. Catalytic conversion of methane to synthesis gas by partial oxidation and CO2 reforming [J]. Advances in Catalysis,2004,48:297-345.
[49] WANG S B,LU G Q. Reforming of methane with carbon dioxide over Ni/Al2O3 catalysts:Effect of nickel precursor [J]. Applied Catalysis A:General,1998,169(2):271-280.
[50] XU J K,ZHOU W,WANG J H,et al. Characterization and anal-ysis of carbon deposited during the dry reforming of methane over Ni/La2O3/Al2O3 catalysts [J]. Chinese Journal of Catalysis,2009,30(11):1076-1084.
[51]  P,ERJAVEC B,et al. Effect of synthesis parameters on morphology and activity of bimetallic catalysts in CO2-CH4 reforming [J]. Chemical Engineering Journal,2012,207:299-307.
P,ERJAVEC B,et al. Effect of synthesis parameters on morphology and activity of bimetallic catalysts in CO2-CH4 reforming [J]. Chemical Engineering Journal,2012,207:299-307.
[52] LIU Z,ZHOU J,CAO K,et al. Highly dispersed nickel loaded on mesoporous silica:One-spot synthesis strategy and high performance as catalysts for methane reforming with carbon dioxide [J]. Applied Catalysis B:Environmental,2012,125:324-330.
[53] HOU Z Y,YASHIMA T. Small amounts of Rh-promoted Ni catalysts for methane reforming with CO2[J]. Catalysis Letters,2003,89(3/4):193-197.
[54] GARC A-DIÉGUEZ M,PIETA I S,HERRERA M C,et al. RhNi nanocatalysts for the CO2 and CO2 H2O reforming of methane [J]. Catalysis Today,2011,172:136-142.
A-DIÉGUEZ M,PIETA I S,HERRERA M C,et al. RhNi nanocatalysts for the CO2 and CO2 H2O reforming of methane [J]. Catalysis Today,2011,172:136-142.
[55] PAWELEC B,DAMYANOVA S,ARISHTIROVA K,et al. Stru-ctural and surface features of PtNi catalysts for reforming of methane with CO2 [J]. Applied Catalysis A:General,2007,323:188-201.
[56] STEINHAUER B,KASIREDDY M R,RADNIK J,et al. Development of Ni-Pd bimetallic catalysts for the utilization of carbon dioxide and methane by dry reforming [J]. Applied Catalysis A:General,2009,366(2):333-341.
[57] SAN-JOSÉ-ALONSO D,JUAN-JUAN J,ILL N-G
N-G MEZ M J,et al. Ni,Co and bimetallic Ni-Co catalysts for the dry reforming of methane [J]. Applied Catalysis A:General,2009,371(1/2):54-59.
MEZ M J,et al. Ni,Co and bimetallic Ni-Co catalysts for the dry reforming of methane [J]. Applied Catalysis A:General,2009,371(1/2):54-59.
[58] WU T,ZHANG Q,CAI W Y,et al. Phyllosilicate evolved hierarchical Ni-and Cu-Ni/SiO2 nanocomposites for methane dry reforming catalysis [J]. Applied Catalysis A:General,2015,503:94-102.
[59] LUCRÉDIO A F,ASSAF J M,ASSAF E M. Methane conversion reactions on Ni catalysts promoted with Rh:Influence of support [J]. Applied Catalysis A:General,2011,400(1/2):156-165.
[60] GARC A-DIÉGUEZ M,PIETA I S,HERRERA M C,et al. Nan-ostructured Pt- and Ni-based catalysts for CO2-reforming of methane [J]. Journal of Catalysis,2010,270(1):136-145.
A-DIÉGUEZ M,PIETA I S,HERRERA M C,et al. Nan-ostructured Pt- and Ni-based catalysts for CO2-reforming of methane [J]. Journal of Catalysis,2010,270(1):136-145.
[61] MA Q X,SUN J,GAO X H,et al. Ordered mesoporous alumina-supported bimetallic Pd-Ni catalysts for methane dry reforming reaction [J]. Catalysis Science & Technology,2016,6:6542-6550.
[62] WANG L,LI D,KOIKE M,et al. Catalytic performance and cha-racterization of Ni-Co catalysts for the steam reforming of biomass tar to synthesis gas[J]. Fuel,2013,112:654-661.
[63] KIM S M,ABDALA P M,MARGOSSIAN T,et al. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts[J]. Journal of the American Chemical Society,2017,139(5):1937-1949.
[64] RAY K,SANDUPATLA A S,DEO G. Activity and stability descriptors of Ni based alloy catalysts for dry reforming of methane:A density functional theory study [J]. International Journal of Quantum Chemistry,2021,121(8):e26580.
[65] 郑幼松,邹宗鹏,吕莉,等. 甲烷干重整抗失活镍基催化剂研究进展 [J]. 天然气化工(C1化学与化工),2021,46(6):1-8.
ZHENG Yousong,ZOU Zongpeng,LV Li,et al. Research progress of anti-deactivation nickel based catalysts for dry reforming of methane [J]. Natural Gas Chemical Industry,2021,46(6):1-8.
[66] WANG J Y,FU Y,KONG W B,et al. Investigation of atom-level reaction kinetics of carbon-resistant bimetallic NiCo-reforming catalysts:Combining microkinetic modeling and density functional theory [J]. ACS Catalysis,2022,12:4382-4393.
[67] ZHANG J G,WANG H,DALAI A K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane [J]. Journal of Catalysis,2007,249(2):300-310.
[68] 吴兴亮,吕凌辉,马清祥,等. 甲烷二氧化碳重整镍基催化剂的研究进展 [J]. 洁净煤技术,2021,27(3):129-137.
WU Xingliang,LYU Linghui,MA Qingxiang,et al. Research progress of nickel-based catalysts for methane carbon dioxide reforming [J]. Clean Coal Technology,2021,27(3):129-137.
[69] 徐占林,毕颖丽,甄开吉. 甲烷催化二氧化碳重整制合成气反应研究进展 [J]. 化学进展,2000,12(2):121-130.
XU Zhanlin,BI Yingli,ZHEN Kaiji. Progress in studies of CO2 reforming of methane to synthesis gas[J]. Progress in chemistry,2000,12(2):121-130.
[70] 孙杰,孙春文,李吉刚,等. 甲烷水蒸气重整反应研究进展 [J]. 中国工程科学,2013(2):98-106.
SUN Jie,SUN Chunwen,LI Jigang,et al. Research development of steam methane reforming reactions [J]. Strategic Study of CAE,2013(2):98-106.
[71] BIAN Z F,DAS S,WAI M H,et al. A review on bimetallic Nickel-Based catalysts for CO2 reforming of methane [J]. ChemPhysChem,2017,18(22):3117-3134.
[72] KAWI S,KATHIRASER Y,NI J,et al. Progress in synthesis of highly active and Stable Nickel-Based catalysts for carbon dioxide reforming of methane [J]. ChemSusChem,2015,8(21):3556-3575.
[73] HAN J W,PARK J S,CHOI M S,et al. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane [J]. Applied Catalysis B:Environmental,2017,203:625-632.
[74] CHEN Y G,REN J. Conversion of methane and carbon dioxide into synthesis gas over alumina-supported nickel catalysts. Effect of Ni-Al2O3 interactions [J]. Catalysis Letter,1994,29(1):39-48.
[75] ZHANG S S,YING M,YU J,et al. NixAl1O2-δ mesoporous catalysts for dry reforming of methane:The special role of NiAl2O4 spinel phase and its reaction mechanism [J]. Applied Catalysis B:Environmental,2021,291:120074.
[76] HORV TH A,GUCZI L,KOCSONYA A,et al. Sol-derived AuNi/MgAl2O4 catalysts:Formation,structure and activity in dry reforming of methane [J]. Applied Catalysis A:General,2013,468:250-259.
TH A,GUCZI L,KOCSONYA A,et al. Sol-derived AuNi/MgAl2O4 catalysts:Formation,structure and activity in dry reforming of methane [J]. Applied Catalysis A:General,2013,468:250-259.
[77] WANG H,MILLER J T,SHAKOURI M,et al. XANES and EX-AFS studies on metal nanoparticle growth and bimetallic interaction of Ni-based catalysts for CO2 reforming of CH4 [J]. Catalysis Today,2013,207:3-12.
[78] RUCKENSTEIN E,HU Y H. Carbon dioxide reforming of meth-ane over nickel/alkaline earth metal oxide catalysts [J]. Applied Catalysis A:General,1995 ,133(1):149-161.
[79] RUCKENSTEIN E,HU Y H. Role of support in CO2 reforming of CH4 to syngas over Ni catalysts [J]. Journal of Catalysis,1996,162(2):230-238.
[80] FERREIRA-APARICIO P,RODR GUEZ-RAMOS I,ANDER-SON J A,et al. Mechanistic aspects of the dry reforming of methane over ruthenium catalysts [J]. Applied Catalysis A:General,2000,202(2):183-196.
GUEZ-RAMOS I,ANDER-SON J A,et al. Mechanistic aspects of the dry reforming of methane over ruthenium catalysts [J]. Applied Catalysis A:General,2000,202(2):183-196.
[81] BITTER J H,SESHAN K,LERCHER J A. Mono and bifunction-al pathways of CO2/CH4 reforming over Pt and Rh based catalysts [J]. Journal of Catalysis,1998,176(1):93-101.
[82]  P,BATISTA J,PINTAR A. Efficient catalytic abatement of greenhouse gases:Methane reforming with CO2 using a novel and thermally stable Rh-CeO2 catalyst [J]. International Journal of Hydrogen Energy,2012,37(3):2699-2707.
P,BATISTA J,PINTAR A. Efficient catalytic abatement of greenhouse gases:Methane reforming with CO2 using a novel and thermally stable Rh-CeO2 catalyst [J]. International Journal of Hydrogen Energy,2012,37(3):2699-2707.
[83] GRONCHI P,MAZZOCCHIA C,ROSSO R D,et al. Carbon dioxide reaction with methane on La2O3 supported Rh catalysts [J]. Energy Conversion and Management,1995,36(6):605-608.
[84] FERREIRA-APARICIO P,GUERRERO-RUIZ A,RODR GUEZ-RAMOS I. Comparative study at low and medium reaction temperatures of syngas production by methane reforming with carbon dioxide over silica and alumina supported catalysts [J]. Applied Catalysis A:General,1998,170(1):177-187.
GUEZ-RAMOS I. Comparative study at low and medium reaction temperatures of syngas production by methane reforming with carbon dioxide over silica and alumina supported catalysts [J]. Applied Catalysis A:General,1998,170(1):177-187.
[85] PAKHARE D,SPIVEY J. A review of dry(CO2) reforming of methane over noble metal catalysts [J]. Chemical Society Reviews,2014,43(22):7813-7837.
[86] DAS S,SENGUPTA M,PATEL J,et al. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles [J]. Applied Catalysis A:General,2017,545:113-126.
[87] GRONCHI P,CENTOLA P,DEL ROSSO R. Dry reforming of CH4 with Ni and Rh metal catalysts supported on SiO2 and La2O3[J]. Applied Catalysis A:General,1997,152(1):83-92.
[88] JAFARBEGLOO M,TARLANI A,MESBAH A W,et al. One-pot synthesis of NiO-MgO nanocatalysts for CO2 reforming of methane:The influence of active metal content on catalytic performance [J]. Journal of Natural Gas Science and Engineering,2015,27:1165-1173.
[89] WANG S B,LU G Q. A comprehensive study on carbon dioxide reforming of methane over Ni/γ-Al2O3 catalysts[J]. Industrial & Engineering Chemisty Research,1999,38(7):2615-2625.
[90] JANG W J,JEONG D W,SHIM J O,et al. H2 and CO production over a stable Ni-MgO-Ce0.8Zr0.2O2 catalyst from CO2 reforming of CH4 [J]. International Journal of Hydrogen Energy,2013,38(11):4508-4512.
[91] ABDULRASHEED A,JALIL A A,GAMBO Y,et al. A review on catalyst development for dry reforming of methane to syngas:Recent advances [J]. Renewable and Sustainable Energy Reviews,2019,108:175-193.
[92] MAKRI M M,VASILIADES M A,PETALLIDOU K C,et al. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5 wt% Ni/Ce1-xMxO2-δ(M=Zr4 ,Pr3 ) catalysts [J]. Catalysis Today,2016,259:150-164.
[93] METTE K,KÜHL S,TARASOV A,et al. Redox dynamics of Ni catalysts in CO2 reforming of methane [J]. Catalysis Today,2015,242:101-110.
[94] GAO X Y,ASHOK J,KAWI S. Smart designs of anti-coking and anti-sintering Ni-based catalysts for dry reforming of methane:A recent review [J]. Reactions,2020,1(2):162-194.
[95] LIU Z Y,GRINTER D C,LUSTEMBERG P G,et al. Dry reform-ing of methane on a highly-active Ni-CeO2 catalyst:E ects of metal-support interactions on C-H bond breaking [J]. Angewandte Chemie International Edition,2016,55(26):7455-7459.
ects of metal-support interactions on C-H bond breaking [J]. Angewandte Chemie International Edition,2016,55(26):7455-7459.
[96] LUSTEMBERG P G,RAM REZ P J,LIU Z Y,et al. Room-temperature activation of methane and dry reforming with CO2 on Ni-CeO2(111) surfaces:E
REZ P J,LIU Z Y,et al. Room-temperature activation of methane and dry reforming with CO2 on Ni-CeO2(111) surfaces:E ect of Ce3 sites and metal-support interactions on C—H bond cleavage [J]. ACS Catalysis,2016,6(12):8184-8191.
ect of Ce3 sites and metal-support interactions on C—H bond cleavage [J]. ACS Catalysis,2016,6(12):8184-8191.
[97] ZHANG Q L,ZHANG T F,SHI Y Z,et al. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane [J]. Journal of CO2 Utilization,2017,17:10-19.
[98] ZHANG G J,LIU J W,XU Y,et al. Ordered mesoporous Ni/Si-lica-carbon as an efficient and stable catalyst for CO2 reforming of methane [J]. International Journal of Hydrogen Energy,2019,44(10):4809-4820.
[99] LI Z W,DAS S,HONGMANOROM P,et al. Silica-based micro- and mesoporous catalysts for dry reforming of methane [J]. Catalysis Science & Technology,2018,8(11):2763-2778.
[100] ZHANG Q L,SUN M H,NIN G P,et al. E ect of thermal induction temperature on re-dispersion behavior of Ni nanoparti-cles over Ni/SBA-15 for dry reforming of methane [J]. Applied Surface Science,2019,469:368-377.
ect of thermal induction temperature on re-dispersion behavior of Ni nanoparti-cles over Ni/SBA-15 for dry reforming of methane [J]. Applied Surface Science,2019,469:368-377.
[101] ZHANG S H,MURATSUGU S,ISHIGURO N,et al. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane [J]. ACS Catalysis,2013,3(8):1855-1864.
[102] MA Q X,HAN Y X,WEI Q H,et al. Stabilizing Ni on bimodal mesoporous-macroporous alumina with enhanced coke tolera-nce in dry reforming of methane to syngas [J]. Journal of CO2 Utilization,2020,35:288-297.
[103] LI Z W,LI M,BIAN Z F,et al. Design of highly stable and selective core/yolv-shell nanocatalysts:A review [J]. Applied Catalysis B:Environmental,2016,188:324-341.
[104] 陈吉祥,王日杰,张继炎,等. 甲烷与二氧化碳重整制取合成气研究进展 [J]. 天然气化工,2003,28(6):32-37.
CHENG Jixiang,WANG Rijie,ZHANG Jiyan,et al. Progress of study on carbon dioxide reforming of methane to synthesis gas[J]. Natural Gas Chemical Industry,2003,28(6):32-37.
[105] 许峥,李玉敏,张继炎,等. 甲烷二氧化碳重整制合成气的镍基催化剂性能 Ⅱ. 碱性助剂的作用 [J]. 催化学报,1997,18(5):364-367.
XU Zheng,LI Yumin,ZHANG Jiyan,et al. Performance of supported nickel catalyst for reforming of CH4 with CO2 to syngas Ⅱ. Effect of basic promoters [J]. Chinese Journal of Catalysis,1997,18(5):364-367.
[106] ALIPOUR Z,REZAEI M,MESHKANI F. Effect of Ni loadings on the activity and coke formation of MgO-modified Ni/Al2O3 nanocatalyst in dry reforming of methane [J]. Journal of Energy Chemistry,2014,23(5):633-638.
[107] DIAS J A C,ASSAF J M. Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane [J]. Catalysis Today,2003,85(1):59-68.
[108] HOU Z Y,YOKOTA O,TANAKA T,et al. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2 [J]. Applied Catalysis A:General,2003,253(2):381-387.
[109] 何素芳. 流化床反应器中用于甲烷二氧化碳氧化重整制合成气反应Ni/SiO2基催化剂的研究 [D]. 杭州:浙江大学,2007.
[110] SUN N N,WEN X,WANG F,et al. Catalytic performance and characterization of NiO-CaO-ZrO2 catalysts for dry reforming of methane [J]. Applied Surface Science,2011,257(21):9169-9176.
[111] AGHAMOHAMMADI S,HAGHIGHI M,MALEKI M,et al.Seq-uential impregnation vs. sol-gel synthesized Ni/Al2O3-CeO2 nanocatalyst for dry reforming of methane:Effect of synthesis method and support promotion [J]. Molecular Catalysis,2017,431:39-48.
[112] YANG W W,LIU H M,LI Y M,et al. CO2 reforming of methane to syngas over highly-stable Ni/SBA-15 catalysts prepared by P123-assisted method [J]. International Journal of Hydrogen Energy,2016,41(3):1513-1523.
[113] XU L L,SONG H L,CHOU L J. Carbon dioxide reforming of methane over ordered mesoporous NiO-MgO-Al2O3 composite oxides [J]. Applied Catalysis B:Environmental,2011,108/109:177-190.
[114] WU J C S,CHOU H C. Bimetallic Rh-Ni/BN catalyst for meth-ane reforming with CO2 [J]. Chemical Engineering Journal,2009,148(2/3):539-545.
[115] GRANT J T,CARRERO C A,GOELTL F,et al. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts [J]. Science,2016,354:1570-1573.
[116] CHEN Y,CHEN T H,LIU H B,et al. High catalytic perform-ance of the Al-promoted Ni/Palygorskite catalysts for dry reforming of methane [J]. Applied Clay Science,2020,188:105498.
[117] VAKILI R,GHOLAMI R,STERE C E,et al. Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts [J]. Applied Catalysis B:Environmental,2020,260:118195.
[118] MA Q X,WANG D,WU M B,et al. Effect of catalytic site position:Nickel nanocatalyst selectively loaded inside or outside carbon nanotubes for methane dry reforming [J]. Fuel,2013,108:430-438.
[119] ZHENG X G,TAN S Y,DONG L C,et al. Plasma-assisted catalytic dry reforming of methane:Highly catalytic performance of nickel ferrite nanoparticles embedded in silica [J]. Journal of Power Sources,2015,274:286-294.
[120] WANG F Q,SHI X H,ZHANG C X,et al. Effects of non-uniform porosity on thermochemical performance of solar driven methane reforming [J]. Energy,2020,191:116575
[121] ZHU M,SONG Y H,CHEN S Y,et al. Chemical looping dry reforming of methane with hydrogen generation on Fe2O3/Al2O3 oxygen carrier [J]. Chemical Engineering Journal,2019,368:812-823.
[122] FABI N-ANGUIANO J A,MENDOZA-SERRATO C G,G
N-ANGUIANO J A,MENDOZA-SERRATO C G,G M EZ-Y
M EZ-Y
 EZ C,et al. Simultaneous CO2 and O2 separation coupled to oxy-dry reforming of CH4 by means of a ceramic-carbonate membrane reactor for in situ syngas production [J]. Chemical Engineering Science,2019,210:115250.
EZ C,et al. Simultaneous CO2 and O2 separation coupled to oxy-dry reforming of CH4 by means of a ceramic-carbonate membrane reactor for in situ syngas production [J]. Chemical Engineering Science,2019,210:115250.
[123] KIM S,RYI S,LIM H. Techno-economic analysis (TEA) for CO2 reforming of methane in a membrane reactor for simultaneous CO2 utilization and ultra-pure H2 production [J]. International Journal of Hydrogen Energy,2018,43(11):5881-5893.
Research progress in carbon deposition resistance of nickel-based catalysts for carbon dioxide reforming of methane

移动阅读
MA Qingxiang,ZHANG Jing,WANG Yijie,et al.Research progress in carbon deposition resistance of nickel-based catalysts for carbon dioxide reforming of methane[J].Clean Coal Technology,2022,28(5):14-28.