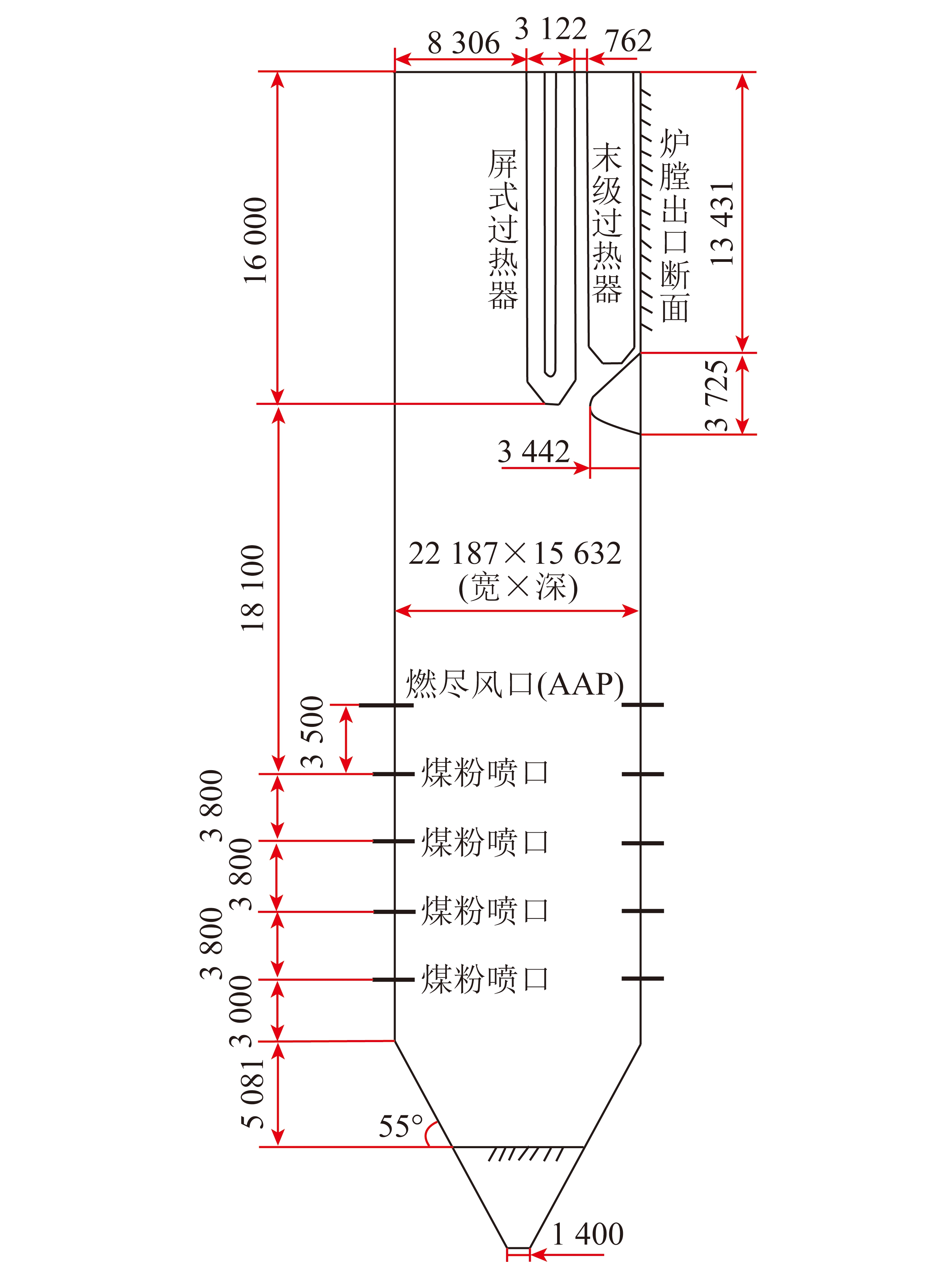
非牛顿熔渣冷却析晶行为研究进展
0 引 言
由于我国富煤贫油少气的能源结构,煤炭清洁高效利用是稳定经济、保障能源安全和低污染排放的关键[1-2]。清洁能源安全稳定供应和经济发展与环境保护的突出矛盾使煤化工技术引起广泛关注。煤化工是以煤炭为原料,通过干馏、气化及液化等方式生产各类化工原料[3]。其中煤气化技术是煤转化为清洁能源燃料(CTL)和合成天然气(SNG)的最有效方式。煤气化技术在世界范围内得到广泛发展。最常用的煤气化工艺是流化床、固定床和气流床[4]。由于气流床气化炉的气化温度高(1 200~1 600 ℃)、原料适应性强及容量大,应用广泛。主要气流床气化技术包括两段式干粉气化、清华炉、多喷嘴、GE Texaco、Shell、GSP等技术[4-5]。
在气化炉中,煤颗粒中碳转化为合成气,煤中矿物质转化为灰分。由于其矿物成分的熔化和反应,灰烬在高温高压条件下变成液态熔渣[6]。在出渣温度下,为确保顺利排渣,避免损坏耐火材料,一般认为炉渣黏度应在2.5~25.0 Pa·s[7-8]。黏度低于25.0 Pa·s的炉渣在重力作用下沿气化炉内壁流动,排入渣池。黏度超过25 Pa·s时,熔渣堵塞出渣口。一旦发生堵塞,必须提高气化炉运行温度,以熔化堵塞熔渣,有时甚至需要关闭气化炉,通过机械方式清除炉渣。然而黏度降至2.5 Pa·s,由于黏度过低可能导致耐火材料壁过度磨损[7-9]。炉内温度高于液相线温度Tliq时,炉渣为均质液体,其黏度取决于结构,即炉渣网络的聚合度[6,10]。熔渣在冷却过程中,部分固态晶体从熔体中析出。晶体增长到一定比例时,熔渣流动特性突变,由牛顿流体转变成非牛顿流体,造成气化炉排渣不畅无法正常运行。煤灰渣中的结晶行为受煤化学组分、温度及冷却速率影响[11-15]。因此,研究煤气化渣的结晶行为,有助于实现炉内液态渣层沿程流动的黏度变化预测,对于优化工程中液态排渣炉内熔渣流动有重要指导意义,可大幅提高装备的可靠性。
笔者综述了煤气化渣的结晶特性,总结了熔渣结晶常用方法,分析了不同方法适用范围和优缺点。讨论了碱性组分(CaO、MgO)、两性组分(Al2O3)及SiO2/Al2O3、CaO/SiO2对熔渣结晶行为的影响,总结了冷却速率和等温温度对熔渣结晶的影响。对于熔渣析出的不同种类晶相,选取熔渣中几种常见晶体(钙长石、黄长石和尖晶石)的生长特性,研究晶体对熔渣流变特性的影响。最后提出非牛顿熔渣的析晶行为的研究方向。
1 结晶研究方法
过去20 a中,熔渣结晶行为研究不断完善,主要包括差示扫描量热法(DSC)[16-17]、X射线衍射(XRD)[18]和高温X射线衍射(HT-XRD)[19]、扫描电子显微镜(SEM-EDS)、热电偶技术(SHTT、DHTT)[20-21]、高温共聚焦扫描激光显微镜(CSLM)[22-23]和FactSage热力学计算[24]。这些研究方法所得结晶信息及优缺点见表1。
表1 硅酸盐渣结晶行为常用研究方法
Table 1 Common methods for studying the crystallization behavior of slag

1.1 差示扫描量热法
DSC是在固定的温控程序下,通过测量输入样品与参比物的功率差,得到热量与温度的关系[16-17]。通过DSC曲线可获得玻璃化转变温度、液相线温度、玻璃相和晶相的潜热等信息[26]。此外,DSC方法是固体物质反应动力学的重要研究手段,结晶动力学信息可使用Kissinger方法求解得到[27],根据无机组分加热或冷却热分析曲线求解得到非等温动力学活化能[28-29]。目前,DSC已广泛用于煤灰渣、高炉渣和保护渣等硅酸盐渣系结晶行为研究[14,30-31]。然而,对于结晶潜热较小的熔渣会导致DSC无法检测到结晶峰[32],DSC冷却/加热速率范围小。
1.2 X射线衍射法
XRD是材料表征的常用方法。目前,主要将XRD与淬冷方法结合得到熔渣高温条件下的晶体状态[28,33-35]。通过分析炉渣的XRD图谱,可得到炉渣的晶体类型。晶体相的衍射峰尖锐,而玻璃相衍射峰比较平缓。由于淬冷试验是熔渣某一时刻的快照,XRD试验无法得到冷却过程中的结晶温度范围和临界冷却速率。
高温XRD可原位测量熔渣在升温和降温过程中的晶相变化。WAANDERS等[36]采用高温XRD测量了煤灰升温过程中组分变化,发现灰渣中矿物结晶度随温度升高而逐渐降低。SUZUKI等[19]通过高温X射线衍射仪原位分析了液态熔渣的冷却析晶过程,得到了不同温度下熔渣的晶体类型。
1.3 扫描电子显微镜(SEM-EDS)
扫描电子显微镜(SEM-EDS)已广泛应用于观察晶体微观形貌、确定元素比例等。通过观察淬冷后的熔渣,可得到晶体形状、大小和比例等详细信息,再结合EDS可确定晶体种类。然而,SEM-EDS试验无法原位观察熔渣的冷却析晶过程。
1.4 热电偶技术(HTT)
基于ORDWAY[37]和WELCH[38]装置原理,KASHIWAYA等[20-21]建立了单/双热电偶技术(SHTT/DHTT)。SHTT和DHTT区别为热电偶数量不同。凭借较高加热/冷却速率及原位观测优势,单热电偶技术(SHTT)成为观察结晶现象首选工具之一[13,25,39]。
SHTT结构简单,精度高。SHTT试验单元、热电偶驱动器和视频捕获系统的试验装置示意[20,25]如图1所示。炉渣样品可直接在直径2 mm的B型Pt-10% Rh热电偶尖端熔化。通常使用纯CaF2校准SHTT温度,炉渣成分均匀性对于结果准确性至关重要。SHTT加热速率高达1 800 K/min[40],最大冷却速率高达6 000 K/min[13],便于研究不同冷却速率和等温温度下熔渣的结晶行为。通过分析SHTT图像随时间、温度和组成变化,可获得熔渣的时间-温度-转变图(TTT)和连续-冷却-转变图(CCT)。此外,对SHTT结晶图像进行处理可得到结晶比例和晶体生长速率等信息[14]。然而,SHTT无法观察到晶体形貌,且对熔渣透明度要求较高。

图1 SHTT装置示意[25]
Fig.1 Schematic diagram of the SHTT[25]
1.5 高温共焦激光扫描显微镜法(CSLM)
CSLM具有原位可视化、高稳定和高精度等特点,是一种先进的获得样品变化和生长的测试方法[22-23]。目前,CSLM已广泛用于原位观察熔渣的相变冷却过程和结晶行为。CSLM试验装置[41]如图2所示,CSLM采用红外加热和氦气冷却可实现高加热速率(1 000 ℃/min)和冷却速率(6 000 ℃/min),温度精度高达0.1 ℃[42-44]。试验过程中,先用卤素红外加热灯将熔渣加热至熔融状态,然后通过调节冷却气体(氦气)注入腔室流量,使熔渣经受各种冷却条件。此外,通过显微镜摄像系统同步获取并记录晶体生长过程。CSLM方法可获得CCT图和TTT图,原位观察晶体形成和生长。然而,CSLM试验只能检测到炉渣表面的结晶,导致不平整液面出现黑色阴影,影响观察。此外,CSLM试验所需样品量很小,要求样品具有很好的均匀性。

图2 CSLM原理[41]
Fig.2 CSLM schematic diagram[41]
1.6 Factsage热力学计算
2001年,F*A*C*T/FACT-Win和ChemSage两个热化学软件包合并,建立了FactSage。FactSage热力学软件由信息、计算和操作模块3部分组成,可访问和操作化合物和溶液数据库。通过FactSage热力学计算可得到复杂氧化物熔渣吉布斯自由能最小化下的相平衡以及黏度[24]。目前,已有很多学者利用FactSage软件研究熔渣的流动特性、矿相组成和熔融特性[45-48]。然而,由于FactSage以相平衡为基础,会出现预测的相图中矿物种类较多、晶体比例偏大等问题。
2 结晶的影响因素
近几年,熔渣结晶特性研究较多,发现熔渣中晶体生长受组分、温度及冷却速率等多因素影响[11-15]。同时熔渣冷却条件(等温温度、冷却速率)也影响熔渣结晶行为。组分和冷却条件对炉渣结晶行为的影响[13,49-62]见表2。
表2 组分和冷却条件对炉渣结晶行为的影响
Table 2 Effect of composition and cooling conditions on the crystallization behavior of slag

2.1 组分对结晶的影响
不同成分对熔渣的影响不同,酸性氧化物如SiO2会导致高聚合度,而碱性氧化物如MgO、FeO和CaO会降低聚合度[6,63-64]。Al2O3是一种两性氧化物,在酸性环境下表现为碱性氧化物,在碱性环境下表现为酸性氧化物,具体取决于炉渣总体成分[65]。许多学者通过改变熔渣中主要成分(CaO、MgO、Al2O3 和SiO2/Al2O3、CaO/SiO2等)比例探究组分对熔渣结晶行为的影响,发现熔渣组分变化会显著影响析晶温度、晶体种类和晶体生长速率等结晶行为。以下总结了主要成分对熔渣结晶行为的影响。
2.1.1 CaO和MgO
碱性氧化物CaO在熔体中会破坏熔体骨架结构,增大熔体中离子的扩散率,使熔渣中的无定形玻璃相转变为晶体相,从而提高熔渣结晶能力。KONG等[66]利用FactSage软件计算炉渣中固相含量,发现固相量平均增加速率Rs随CaO含量的增加而增加,该结果基于相平衡的结果,并未考虑结晶动力学的影响。XUAN等[49]利用SHTT研究了CaO含量对煤渣结晶行为的影响,发现随CaO含量增加熔渣结晶温度先上升后下降再上升,晶体比例先上升后下降,如图3所示。这种变化主要由晶体种类变化引起,CaO质量分数在5%~35%时,主要晶体类型为透辉石(CaMgSi2O6)和钙长石(CaAl2Si2O8)。CaO质量分数超过40%时,晶体类型转变为硅酸二钙(Ca2SiO4),结晶起始温度升高,结晶比例下降。SONG等[67]和KONG等[68]也发现了类似现象,CaO质量分数超过一定量时,随CaO增加,熔渣灰熔融温度(AFTs)增加较快。WANG等[50]使用CSLM原位观察CaO含量对硅锰渣结晶行为的影响,发现硅锰渣晶体比例随CaO含量增加而增大,随CaO含量进一步增大,晶体类型由钙长石变为黄长石。综上所述,熔渣中CaO含量变化导致含钙矿物转变,更高的CaO含量使熔体析晶温度升高。目前,大多数CaO对熔渣的影响主要集中在AFT、黏温特性等宏观层面,缺乏结晶动力学的机理研究。

图3 连续冷却条件下不同含量CaO的结晶率[49]
Fig.3 Crystallization rates of different contents of CaO under continuous cooling conditions[49]
XUAN等[51]使用SHTT研究了MgO对合成煤渣结晶行为的影响,没有MgO的熔渣呈玻璃体状态,随MgO含量增加,结晶温度和晶体比例提高。因此,MgO对熔渣结晶行为的影响机理与CaO相似,加入MgO会使熔渣结晶能力增强,熔渣结晶比例增大、临界冷却速率增加、结晶温度升高、成核时间缩短[54],如图4所示。此外,Mg2 进入熔体中会积极参与结晶过程,促进透辉石、尖晶石(spinel)、黄长石(melilite)、斜辉石(clinopyroxene)以及镁硅钙石(Ca3MgSi2O8)等含Mg2 矿物的结晶,晶体类型转变导致熔渣析晶温度升高[51-52,69-70]。REN等[52]证明MgO质量分数由6%增至12%时,高炉渣的结晶起始温度增大。同时钙长石和硅灰石(CaSiO3)转变为黄长石、斜辉石和尖晶石等含镁相。2022年,CHEN等[70]发现随MgO含量增加,高碱性渣结晶温度逐渐降低,结晶速率先降低后升高,且熔渣中晶相由枪晶石(Ca4Si2F2O7)逐渐转变为氟化钙(CaF2)和镁硅钙石(Ca3MgSi2O8)。

图4 MgO含量对熔渣结晶行为的影响[51,54]
Fig.4 Effect of MgO content on the crystallization behavior of slag[51,54]
2.1.2 Al2O3的影响
Al2O3是一种两性氧化物,在不同熔渣中性质不同。SUN等[53]使用SHTT原位观察了不同Al2O3含量的高炉渣冷却相变过程,发现熔渣的临界冷却速率随Al2O3含量增加先增大后减小。WANG等[71]发现随Al2O3含量增加,临界冷却速率降低,临界过冷度增加,这归因于晶体类型由钙镁黄长石(Ca2MgSi2O7)转变为钙铝黄长石(Ca2Al2SiO7)。表明Al2O3在碱性环境下表现为酸性氧化物,抑制熔渣结晶,使临界冷却速率降低,成核时间增加[54,71-73]。Al2O3在酸性环境下表现为碱性氧化物,促进炉渣结晶[53]。Al2O3对矿物的种类也有显著影响,随Al2O3含量增加,晶体类型由钙镁黄长石转变为钙铝黄长石及尖晶石等含Al3 矿物[71,74]。此外,Al2O3对晶体的形貌有显著影响。ZHANG等[75]利用CSLM研究了Ni-Fe熔渣的结晶行为,发现随Al2O3含量增加,晶体尺寸由400 μm以上减小至50 μm以下,结晶时间增大,镍铁渣结晶能力降低,如图5所示。同时,随Al2O3含量增加,初始结晶温度先升高后降低。李刚等[72]采用改进的实时高温原位结晶性能测试仪研究了Al2O3对高碱度保护渣结晶性能的影响,研究表明熔渣中析出的晶体为枪晶石。随Al2O3质量分数增加,熔渣开始结晶温度下降、平均结晶速率减小、晶体比例降低。

图5 不同Al2O3含量的Ni-Fe渣的CSLM图像[75]
Fig.5 CSLM images of Ni-Fe slag with different Al2O3 contents[75]
2.1.3 SiO2/Al2O3
熔渣的聚合度随SiO2含量增加而增加。相比之下,聚合度随低Al2O3含量熔渣中Al2O3含量的增加而增加,然后随Al2O3含量进一步增加而逐渐降低[76]。因此,熔渣结晶能力变化趋势归因于SiO2和Al2O3质量比变化。通常,随SiO2/Al2O3质量比增加,高炉渣和保护渣的结晶能力降低[55-57]。SiO2替换Al2O3后,熔体扩散能力减弱,抑制晶体成核和生长。WANG等[56]采用共聚焦激光扫描显微镜(CLSM)研究了Al2O3/SiO2质量比为0.03~1.10的炉渣结晶行为,绘制了CCT图和TTT图,如图6(a)所示。发现随Al2O3/SiO2质量比增大初始结晶温度升高,结晶孵育时间缩短,晶体类型由钙镁黄长石或镁硅钙石转变为钙铝黄长石。然而,XUAN等[14]通过DSC和SHTT试验研究了不同SiO2/Al2O3质量比的煤渣结晶行为,发现低SiO2熔渣中,适当提高SiO2/Al2O3质量比可提高煤渣结晶能力。随SiO2/Al2O3质量比进一步增加,其结晶能力受到抑制,如图6(b)所示。这是由于高Al2O3熔渣的聚合度很高,削弱了结晶倾向,低迁移速率导致晶体很难析出,因此SiO2/Al2O3一定程度增加会增强熔渣结晶能力。

图6 熔渣的结晶行为随SiO2/Al2O3的变化[14,56]
Fig.6 Change of crystallization behavior of slag with SiO2/Al2O3[14,56]
2.1.4 CaO/SiO2
由于碱性组分和酸性组分对熔渣结晶特性的影响机制不同。二元碱度(CaO/SiO2)用来描述熔体的酸碱特性。许多学者研究了二元碱度对熔渣结晶特性的影响,发现随CaO/SiO2质量比增大,熔渣黏度减小,结晶能力增强[54,58-60]。QIN等[54]发现增大CaO/SiO2将导致高炉渣的临界冷却速率增大、结晶温度升高以及成核时间缩短。其他学者也得到了相同的结论[59-60]。QIU等[58]研究了CaO/SiO2质量比对炉渣性质的影响,发现随CaO/SiO2质量比增加,黄长石增多,钙长石逐渐减少,并有少量硅酸二钙生成。
2.2 冷却条件对结晶的影响
冷却条件对熔渣的结晶行为有显著影响,这是由于晶体的成核和生长需要一定过冷度和孕育时间。许多学者采用先进的SHTT和CSLM探索了等温冷却和连续冷却对晶体生长行为的影响[13,61,77-78]。
2.2.1 冷却速率对结晶的影响
冷却速率会显著影响熔渣开始结晶温度、晶体孕育时间及晶体形貌[79]。对于连续冷却的熔渣,低冷却速率使晶体有足够的孕育时间,导致熔渣开始析晶温度较高,晶体尺寸和比例增大。随冷却速率增大,高冷却速率极大抑制晶体生长,晶体变得更细更小,甚至超过临界冷却速率时表现为玻璃状[80]。XUAN等[13]使用SHTT技术研究了合成煤渣的结晶特性,发现冷却速率在80 ℃/min前对熔渣结晶特性的影响并不显著。高冷却速率极大抑制晶体生长,超过临界冷却速率时煤渣表现为玻璃状。此外高冷却速率导致晶体孵育时间缩短,开始结晶温度降低[61-62],如图7(a)所示。

图7 硅锰渣的CCT和TTT图[61]
Fig.7 CCT and TTT diagram of silicomanganese slag[61]
2.2.2 等温温度对结晶的影响
高温下,晶体生长受驱动力不足限制。温度低于液相线温度,晶体开始生长。LIN等[62]使用SHTT研究了高炉渣的结晶动力学,发现等温冷却条件下高炉渣的临界冷却速率为9 ℃/s,随温度降低,熔渣中晶体的生长速率先增大后减小。这是由于低温区熔体的扩散性能较差,晶体的生长受扩散限制[13]。
WANG等[61]使用共聚焦激光扫描显微镜(CLSM)原位观察等温温度和冷却速率对硅锰渣结晶性能的影响。发现在等温条件下硅锰渣的结晶度随温度降低而增大,如图7(b)所示。XUAN等[13]利用SHTT技术研究合成煤渣结晶特性也观察到了这一现象。汪达等[15]采用单热电偶在线观察系统(SHTT)、图像分析程序等研究等温过程中温度对于人工配制灰渣结晶行为的影响,发现随等温温度降低,晶体孕育时间先减小后增大,晶体尺寸减小。此外熔渣结晶比例先增大再稳定最后减小,这是由于不同温度区间生成晶体类型不同,导致结晶比例在某些温度变化较大。
3 常见气化熔渣晶体的生长特性
许多学者研究了组分和冷却条件对硅酸盐渣结晶行为的影响。发现组分变化直接导致熔渣中晶体类型改变,从而使熔渣结晶行为发生变化。不同晶体的结晶温度范围及生长机理不同,因此研究晶体结晶机理对了解硅酸盐渣结晶特性尤为重要。以往研究中出现的晶体[8,18,50,81-88]见表3。选取常见的钙长石、黄长石和尖晶石3种晶体,总结晶体析晶行为。
表3 文献中灰熔渣试验观察到的晶相
Table 3 Crystalline phases observed in ash slag experiments from the literature

3.1 钙长石的结晶特性
以往SEM和CSLM试验结果表明钙长石形状在不同熔渣中表现为矩形长条状或扁平状[18,34,61],如图8、9所示。XUAN等[18]采用CSLM研究了4种煤渣的结晶特性,发现煤灰渣中析出的钙长石呈细长条状或扁平状。结晶比例达15%~20%时,黏度受严重影响。HE等[89]研究钙长石的结晶动力学,发现钙长石为界面控制的一维生长。然而,WANG等[61]采用CLSM开展了硅锰废渣等温结晶和连续冷却结晶试验,发现钙长石的结晶动力学受温度影响。随温度降低,钙长石晶体生长机理由二维变为一维,熔渣结晶趋势变强。此外,钙长石的结晶受碱性氧化物影响,研究表明钙长石结晶温度随碱性氧化物加入而降低[34]。等温温度对钙长石的结晶也有显著影响。SCHUPSKY等[90]采用SEM发现随等温温度升高,钙长石长度增加。这可能是由于低温下钙长石的生长受扩散限制。

图8 SEM试验中钙长石晶体形态[34,89-90]
Fig.8 Crystal morphology of anorthite in SEM experiments[34,89-90]

图9 SEM和CSLM试验中钙长石晶体形态对比[18,50,61]
Fig.9 Comparison of anorthite crystal morphology in SEM
and CSLM experiments[18,50,61]
3.2 黄长石的结晶特性
黄长石晶体形状表现为柱状或板状结构[50,85,91],如图10所示。不同黄长石晶体(钙镁黄长石和钙铝黄长石)对熔渣结晶特性的影响不同。WANG等[71]使用改进的定向凝固方法研究了Al2O3对高炉渣结晶行为的影响,发现晶体类型由钙镁黄长石转变为钙铝黄长石,导致高炉渣临界平均冷却速率降低,临界过冷度增加。黄长石与钙长石类似,黄长石也是熔渣中常见初生相之一。因此黄长石的结晶会影响熔渣的开始析晶温度、晶体孕育时间等结晶行为[52,56,71,91]。REN等[91]采用SHTT研究了改性高炉渣的结晶行为,飞灰质量分数在5%~20%时,改性高炉渣的初始结晶温度受黄长石结晶影响。飞灰质量分数增至25%时,初始结晶温度受钙长石结晶的影响。此外,黄长石结晶特性受冷却速率、等温温度和气氛等外部条件影响[83,92]。SHEN等[83]利用XRD和SEM分析了煤渣中黄长石的生长行为,发现随冷却速率增加,SEM图像中晶体尺寸在长度和宽度方向上均减小,而同一区域内晶体数量增加。SCHWITALLA等[92]发现钙铝黄长石和钙镁黄长石在不同气氛下的生长速率不同。

图10 不同研究中黄长石的结晶图像[50,83,91]
Fig.10 Crystalline images of melilite from different studies[50,83,91]
3.3 尖晶石的结晶特性
尖晶石晶体表现为八面体形状[89,93-95],如图11所示。尖晶石的生长为扩散控制机制,扩散速率/黏度决定尖晶石生长动力学[69,89]。ILYUSHECHKIN等[88]发现尖晶石的等温结晶动力学强烈依赖熔渣黏度。尖晶石生长受过冷度影响,随过冷度增加,熔渣中尖晶石结晶速率增大,孕育时间缩短,尖晶石结晶形成网状结构[94]。此外,尖晶石结晶也会影响熔渣的开始析晶温度。XUAN等[51]研究了MgO对合成煤渣结晶行为的影响,XRD发现Mg2 积极参与结晶过程,加入MgO导致高温晶相逐渐由钙长石转变为尖晶石。同时SHTT试验发现随MgO含量增加,结晶温度提高。说明尖晶石析晶温度高于钙长石。

图11 不同研究中尖晶石的结晶图像[89,93-95]
Fig.11 Crystalline images of spinel from different studies[89,93-95]
综上所述,钙长石、黄长石和尖晶石3种晶体的生长特性(结晶动力学、晶体形貌和开始结晶温度等)区别显著。3种晶体形状不受组分和冷却条件影响,钙长石表现为长条状,黄长石表现为方块状,尖晶石表现为八面体形状。然而,晶体大小受等温温度和冷却速率的影响。一般来说,低温和高冷却速率不利于晶体生长,晶体尺寸较小。
4 晶体对熔渣流变特性的影响
在液态排渣高温炉中,反应后灰渣以熔融状态沿炉内侧壁向下流动。在熔渣液相线温度之上,熔渣黏度受高温下熔体的结构和温度影响[6,96]。酸性氧化物如SiO2导致高黏度,而碱性氧化物如MgO、FeO和CaO会降低黏度[6,63-64,97-98]。冷却过程中,部分固态晶体会从熔体中析出。晶体可改变熔体组分,且随晶体含量增加,灰渣流动特性由牛顿流体转变成非牛顿流体[99],如图12所示。其黏度(通常称为相对黏度或有效黏度)取决于晶体比例、尺寸和形状等因素[100-102]。结晶渣黏度试验测定和结果解释复杂,渣中结晶析出的固相影响整体黏度和液相组成。以下综述熔渣流变特性的主要影响因素。

图12 结晶对煤气化渣黏温特性的影响
Fig.12 Effect of crystallization on the viscosity-temperature properties of coal gasification slag
4.1 晶体比例
HOY等[103]研究发现熔渣中的晶体对熔渣黏度有显著影响,且不断漂浮的晶体对黏度的影响远大于固定的晶体。随晶体含量增大,熔渣中晶体开始互相影响,且连接在一起。晶体比例为50%时,熔渣表现为非牛顿流体特性。宋文佳[104]利用黏度试验和FactSage热力学计算研究了煤灰样品的黏温特性,发现煤灰中有固体晶体析出(晶体质量分数在10%左右)时,此时温度对应黏温曲线的临界黏度温度。然而,KONG等[105]认为临界黏度温度与固相含量无关,其对应于固相含量(FactSage计算)的突变点。由于气化炉高温高压的运行特点,难以直接观测熔渣在气化炉内的流动结晶行为。由于研究工具限制,许多学者使用FactSage吉布斯自由能最小化计算晶体含量,而FactSage软件基于相平衡计算,造成计算结果无法预测实际的非平衡结晶过程。
最近,XUAN等[84]利用SHTT技术对真实煤灰的结晶特性进行研究,通过图像处理获得固相含量。发现尖晶石比例约为20%时,尖晶石形成网状结构,炉渣黏度急剧增加。HE等[106]研究了煤渣黏度测量过程中尖晶石和钙长石相的等温结晶,发现随晶体生长,熔渣黏度连续增大。并指出尖晶石的结晶增加了液相黏度,钙长石的结晶减小液相黏度。
4.2 颗粒形状
颗粒形状对黏度有显著影响,如球形颗粒对黏度的影响远小于不规则颗粒对黏度的影响[107]。WRIGH等[108-109]对球形颗粒和不规则尖晶石颗粒的研究也验证这一观点。OH等[7]研究煤灰渣发现,树枝状晶体相互连接形成网络结构导致熔渣黏度发生突变。
LIU等[110]利用共聚焦激光扫描显微镜(CLSM)原位观察渣的结晶行为,发现临界固相分数Φc随长径比(2.17~1.43)的减小而增大,如图13(a)所示(BOF为高炉渣样品,xS为BOF和质量x的SiO2混合后样品,xA为BOF和质量x的Al2O3混合后的样品)。MOITRA等[111]研究了不同尺寸和形状的粒子对悬浮液流变行为的影响,发现悬浮液的最大填充分数Φm取决于晶体尺寸和形状分布,如图13(b)所示(s为小球颗粒,S为大球颗粒,e为长径比2、平均长度35 μm的圆柱颗粒,E为长径比6、平均长度122 μm的圆柱颗粒,Ss、Se等为不同颗粒混合的悬浮液,λ为颗粒直径比值,ar为细长颗粒的长径比)。随小颗粒增多,最大填充分数先增大后减小,且含有低长径比组合颗粒的悬浮液最大填充分数最大。这是因为大颗粒产生的空隙更易被低纵横比小颗粒填充。XUAN等[18]采用CSLM研究了4种煤渣在还原气氛下的结晶特性。发现煤渣中的同一种晶体也可以显示不同形状,钙长石表现出细长条状、扁平状。以钙长石为初生相的煤灰渣,结晶率增至15%~20%时,其黏度急剧增加。ILYUSHECHKIN等[112]研究了橄榄石初生相场的2种熔体黏度,发现较大的冷却速率导致细长晶体的形成,熔渣表现出非牛顿行为。缓慢冷却导致熔体中形成多面体形状的晶体,其熔渣表现出牛顿行为。HE等[34]将最大填充分数表示为晶体长径比的函数,并结合液相黏度和固相分数推导出非牛顿煤灰渣的黏度预测模型。MA等[35]发现矿渣中结晶量在20%~ 43%时,矿渣黏度迅速上升,且粒状晶体对黏度的影响大于片状晶体。

图13 颗粒形状对非牛顿流体流变特性的影响[110-111]
Fig.13 Effect of particle shape on the rheological properties
of non-Newtonian fluids[110-111]
4.3 晶体尺寸
在真实熔渣析晶过程中,由于不同晶体形状和复杂的尺寸分布,使研究晶体尺寸分布对黏度的影响更加复杂。大多研究取平均粒径,研究其对黏度的影响。
BRUIJN[113]研究结果表明,对于颗粒大于10 μm的悬浮液,碰撞后颗粒旋转恢复导致额外能量耗散,导致黏度随着颗粒尺寸的增加而增加。WRIGHT等[108-109]对煤灰渣研究也证实这点。ILYUSHECHKI等[114]发现在相对较低的硅铝比(S/A)比下,固体含量达到一定值(通常超过15%)或显著增加晶体尺寸,熔渣黏度急剧增加。然而,LIU等[110]观察到临界固相分数Φc随晶体长径比的增大而降低,然而试验结果表明Φc与晶体尺寸之间无明显规律。
4.4 剪切速率
SAAR等[115]发现平行排列的晶体比随机取向晶体的临界固相分数更高。这说明当固相比例达到一定值时,熔渣会表现出剪切依赖性。
NGUYEN[116]研究了剪切速率对粉煤灰表观黏度的影响,发现熔体表观黏度随剪切速率的增大而减小。SONG等[117]发现熔渣结晶体积分数高于4.70%,随温度降低呈明显的剪切减薄等非牛顿行为,如图14所示。LIU等[118]研究发现晶体含量超过临界固相分数时,出现剪切变薄现象,黏度急剧增加。晶体含量高于临界固相分数时,表观屈服应力与触变流动同时存在,呈现出时间依赖性。认为表观屈服应力归因于骨架固体网络的形成。SEEBOLD等[119]发现过冷度较高时,部分结晶渣表现出伪塑性。

图14 煤灰熔渣的剪切稀化行为[117]
Fig.14 Shear thinning behavior of coal ash slag[117]
不同的炉渣特性,如液相组成、固相浓度和晶体形态,对炉渣黏度的影响不同。黏度测量程序影响含固相煤灰熔渣的流变行为,包括剪切依赖行为、时间依赖行为、屈服应力(表4)。此外,黏度测量试验与结晶试验降温程序的一致性对于认识熔渣的流变行为极其重要。然而,目前对含有多分散粒子的非牛顿流体的流变特性研究还不充分,且剪切行为使晶体破碎,熔渣变为更复杂的非牛顿流体。因此,目前还没有考虑到所有影响因素的非牛顿熔渣的黏度预测模型。
表4 晶体对煤灰流动性的影响
Table 4 Effect of crystals on the fluidity of coal ash

续表

5 结语及展望
煤气化渣的流动行为严重影响气化炉平稳运行,熔渣中大量析出晶体时,熔渣黏度迅速增大,因此其结晶特性受广泛关注。
1)在多种晶体研究方法中,原位观察由于数据可靠性及高冷却/加热速率优势,已成为观察高温熔渣结晶过程的常用方法,可清晰展示晶体生长过程。然而,在线观察的SHTT方法无法观测晶体形貌,且温度精度较差;CSLM试验控制精度较高,能较清晰观察晶体生长过程,但受制于观察范围,只能观察某部分区域结晶及晶体三维生长。寻找定量确定晶体的三维表征方法有利于更深入研究熔渣的结晶特性。
2)组分和冷却条件对气化熔渣的结晶行为有显著影响,而析出晶相的变化是结晶行为突变的根本原因。晶体形状与晶体种类有关,不同熔渣组分析出的晶体种类各异。此外,晶体大小受等温温度和冷却速率影响。一般来说,高温和低冷却速率有利于晶体生长,晶体尺寸较大。但目前熔渣组分与结晶动力学的对应关系还不明确。在预测方法上,目前熔渣的计算多依靠FactSage软件,但该方法可实现热力学平衡状态下的固相比例,与实际析出的晶体种类有一定差异,而且基于平衡的计算无法揭示动力学特性,未来需建立考虑动力学影响的晶体生长模型。
3)熔渣的流变特性受液相组成、固相浓度、晶体形态及剪切速率等多重因素影响,剪切行为使晶体破碎,熔渣变为多分散粒子的非牛顿流体。目前由于缺乏高温熔渣晶体动力学特性和对应条件下的黏度数据,还无法定量确定晶体对流变行为的影响。因此,熔渣晶体对流变行为的影响规律还有待进一步研究。
[1] ZHAO L T, LIU Z T, CHENG L. How will China′s coal industry develop in the future? A quantitative analysis with policy implications[J].Energy, 2021,235:121406.
[2] XU J,YANG Y,LI Y W. Recent development in converting coal to clean fuels in China[J]. Fuel,2015,152:122-130.
[3] BELL D A,TOWLER B F,FAN M.Chapter 4.Gasifiers,in: Coal gasification and its applications[M]. Boston: William Andrew Publishing,2011: 73-100.
[4] 玄伟伟. 非牛顿煤灰熔渣结晶过程及动力学实验研究[D].北京:清华大学, 2015:1-2.
[5] KONG L,BAI J,LI W. Viscosity-temperature property of coal ash slag at the condition of entrained flow gasification: A review[J]. Fuel Processing Technology,2021,215:106751.
[6] VARGAS S,FRANDSEN F J,DAM-JOHANSEN K. Rheological properties of high-temperature melts of coal ashes and other silicates[J]. Progress in Energy and Combustion Science,2001,27(3):237-429.
[7] OH M S,BROOKER D D,DE PAZ E F,et al. Effect of crystalline phase formation on coal slag viscosity[J]. Fuel Processing Technology,1995,44(1/3):191-199.
[8] YUAN H,LIANG Q,GONG X. Crystallization of coal ash slags at high temperatures and effects on the viscosity[J]. Energy &Fuels,2012,26(6):3717-3722.
[9] JAN W N. Viscosity and phase transformation in coal ash slags near and below the temperature of critical viscosity[J]. Energy &Fuel,1994,36(2):1324-1336.
[10] LIN X,LIU J,IDETA K,et al. Cation induced microstructure and viscosity variation of molten synthetic slag analyzed by solid-state NMR[J]. Fuel,2020,267:117310.
[11] DING B,WANG H,ZHU X,et al. Prediction on crystallization behaviors of blast furnace slag in a phase change cooling process with corrected optical basicity[J]. Fuel,2018,223:360-365.
[12] SHEN Z,HUA X,LIANG Q,et al. Reaction,crystallization and element migration in coal slag melt during isothermal molten process[J]. Fuel,2017,191:221-229.
[13] XUAN W,WHITTY K J,GUAN Q,et al. Influence of isothermal temperature and cooling rates on crystallization characteristics of a synthetic coal slag[J]. Fuel,2014,137:193-199.
[14] XUAN W,WHITTY K J,GUAN Q,et al.Influence of SiO2/Al2O3 on crystallization characteristics of synthetic coal slags[J]. Fuel,2015,144:103-110.
[15] 汪达,王倩,张建胜. 不同温度和冷却速率下高温灰渣的结晶行为[J]. 化工学报,2018,69(5):2183-2190.
WANG Da,WANG Qian,ZHANG Jiansheng. Crystallization behaviors of molten ash slag under different temperatures and cooling rates[J]. CIESC Journal,2018,69(5): 2183-2190.
[16] FREDERICCI C,ZANOTTO E D,ZIEMATH E C. Crystallization mechanism and properties of a blast furnace slag glass[J]. Journal of Non-Crystalline Solids,2000,273(1):64-75.
[17] XUAN W,WANG Q,ZHANG J,et al. Influence of silica and alumina (SiO2 Al2O3) on crystallization characteristics of synthetic coal slags[J]. Fuel,2017,189:39-45.
[18] XUAN W,WANG H,XIA D. In-situ observation of crystallization inside coal slags and influence of crystals on flow behavior[J]. Fuel,2019,251:242-248.
[19] SUZUKI M,SERIZAWA H,UMESAKI N. Phase identification of crystal precipitated from molten CaO-SiO2-FeOx-P2O5 slag by high temperature in-situ X-ray diffraction[J]. Iron and Steel Institute of Japan International,2020,60(12):2765-2772.
[20] KASHIWAYA Y,CICUTTI C E,CRAMB A W,et al. Developm-ent of double and single hot thermocouple technique for in situ observation and measurement of mold slag crystallization[J]. Iron and Steel Institute of Japan International,1998,38(4):348-356.
[21] KASHIWAYA Y,CICUTTI C E,CRAMB A W. An Investigation of the crystallization of a continuous casting mold slag using the single hot thermocouple technique[J]. ISIJ International,1998,38(4):357-365.
[22] WANG L,LI J,YANG S,et al . Coarsening behavior of particles in Fe-O-Al-Ca melts[J]. Scientific Reports,2019,9(1):3670.
[23] XUAN W,ZHANG Y,ZHANG J. Chemistry variation of slag and the layered characteristics of deposits in an industrialized entrained-flow gasifier system with radiant syngas cooler[J]. Energy,2022,260:124942.
[24] BALE C W,CHARTRAND P,DEGTEROV S A,et al. FactSage thermochemical software and databases[J]. Computer Coupling of Phase Diagrams and Thermochemistry,2002,26(2):189-228.
[25] WANG Z,SOHN I. A review of in situ observations of crystallization and growth in high temperature oxide melts[J]. Journal of metals,2018,70(7):1210-1219.
[26] DING B,WANG H,ZHU X,et al. Phase change cooling and crystallization characteristics of blast furnace slags with various MgO/Al2O3 ratios[J]. Energy &Fuels,2017,31(9):10212-10221.
[27] BLAINE R L,KISSINGER H E. Homer kissinger and the kissi-nger equation[J]. Thermochimica Acta,2012,540:1-6.
[28] WU J,LI Z,HUANG Y,et al . Crystallization behavior and properties of K2O-CaO-Al2O3-SiO2 glass-ceramics[J]. Ceramics International,2013,39(7):7743-7750.
[29] FRANCIS A A,RAWLINGS R D,SWEENEY R,et al.Crystallization kinetic of glass particles prepared from a mixture of coal ash and soda-lime cullet glass[J]. Journal of Non-Crystalline Solids,2004,333(2):187-193.
[30] GAN L,ZHANG C,SHANGGUAN F,et al . A differential scanning calorimetry method for construction of continuous cooling transformation diagram of blast furnace slag[J]. Metallurgical and Materials Transactions B,2012,43(3):460-467.
[31] MALDONADO Y G,HUMBERTO Castillejos E A. A new method for estimating the isothermal devitrification and crystallization of mold powder slags from non-isothermal DSC data[J]. Materials &Design,2015,83:728-735.
[32] SHI C B,SEO M D,CHO J W,et al.Crystallization characteristics of CaO-Al2O3-based mold flux and their effects on in-mold performance during high-aluminum TRIP steels continuous casting[J].Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science,2014,45:1081-1097.
[33] GE Z,KONG L,BAI J,et al. Effect of CaO/Na2O on slag visco-sity behavior under entrained flow gasification conditions[J]. Fuel Processing Technology,2018,181:352-360.
[34] HE C,ILYUSHECHKIN A,BAI J,et al. Viscosity and crystallisation behaviour of coal ash slag from the primary phase of anorthite[J]. Fuel Processing Technology,2021,213:106680.
[35] MA H,JIAO K,ZHANG J,et al. Viscosity of CaO-MgO-Al2O3-
SiO2-TiO2-FeO slag with varying TiO2 content: The effect of crystallization on viscosity abrupt behavior[J]. Ceramics International,2021,47(12):17445-17454.
[36] WAANDERS F B,VAN DYK J C,PRINSLOO C J. The characterisation of three different coal samples by means of various analytical techniques[J]. Hyperfine Interactions,2009,190(1):109-114.
[37] ORDWAY F. Techniques for growing and mounting small single crystals of refractory compounds[J]. Journal of research of the National Bureau of Standards,1952,48:152.
[38] WELCH J H. A simple microscope attachment for observing high-temperature phenomena[J]. Journal of Scientific Instruments,1954,31(12):458.
[39] KÖLBL N,MARSCHALL I,HARMUTH H. High-temperature investigation of mould slag crystallization by single and double hot thermocouple techniques[J]. Journal of Iron and Steel Research International,2019,26(4):345-354.
[40] YANG J,ZHANG J,SASAKI Y,et al. In-situ study of crystallisation behaviour of CaO-SiO2-Na2O-B2O3-TiO2-Al2O3-MgO-Li2O fluorine-free mould fluxes with different CaO/SiO2 ratios[J]. Iron and Steel Institute of Japan International,2016,56:574-583.
[41] JUNG S S,SOHN I. Crystallization behavior of the CaO-Al2O3-
MgO system studied with a confocal laser scanning microscope[J]. Metallurgical and Materials Transactions B,2012,43(6):1530-1539.
[42] LIU J,GUO M,JONES P T,et al.In situ observation of the direct and indirect dissolution of MgO particles in CaO-Al2O3-SiO2-based slags[J]. Journal of the European Ceramic Society,2007,27(4):1961-1972.
[43] NAKANO J,SRIDHAR S,MOSS T,et al.Crystallization of sy-nthetic coal-petcoke slag mixtures simulating those encountered in entrained bed slagging gasifiers[J]. Energy &Fuels,2009,23(10):4723-4733.
[44] ORRLING C,SRIDHAR S,CRAMB A W. In situ observation of the role of alumina particles on the crystallization behavior of slags[J]. ISIJ International,2000,40(9):877-885.
[45] LI Y,LI F,MA M,et al. Prediction of ash flow temperature based on liquid phase mass fraction by FactSage[J]. Journal of the Energy Institute,2020,93(6):2228-2231.
[46] LI H,YOSHIHIKO N,DONG Z,et al. Application of the FactSa-ge to predict the ash melting behavior in reducing conditions[J]. Chinese Journal of Chemical Engineering,2006,14(6):784-789.
[47] VAN DYK J C,WAANDERS F B,BENSON S A,et al.Viscosity predictions of the slag composition of gasified coal,utilizing FactSage equilibrium modelling[J]. Fuel,2009,88(1):67-74.
[48] LIAO J,ZHAO B. Effects of Al2O3 and "Cr2O3" on phase equilibria of the system "FeO"-MgO-SiO2 at iron saturation[J]. Computer Coupling of Phase Diagrams and Thermochemistry,2021,75:102343.
[49] XUAN W,WHITTY K J,GUAN Q,et al.Influence of CaO on cr-ystallization characteristics of synthetic coal slags[J]. Energy and Fuels,2014,28(10):6627-6634.
[50] WANG W,DAI S,ZHANG T,et al.The effect of CaO on the crystallization properties and viscosity of synthetic silicomanganese waste slag for mineral wool production[J]. Journal of Cleaner Production,2021,288:125603.
[51] XUAN W,ZHANG J,XIA D. The influence of MgO on the crystallization characteristics of synthetic coal slags[J]. Fuel,2018,222:523-528.
[52] REN Q Q,ZHANG Y Z,LONG Y,et al.Investigation on the effect of MgO content on the crystallization behavior of synthetic BF slag[J]. Materials and Manufacturing Processes,2018,33(15):1654-1660.
[53] SUN Y,SHEN H,WANG H,et al.Experimental investigation and modeling of cooling processes of high temperature slags[J]. Energy,2014,76:761-767.
[54] QIN Y,LV X,ZHANG J. Effect of composition on the crystallisation behaviour of blast furnace slag using single hot thermocouple technique[J]. Ironmaking &Steelmaking,2017,44(1):23-27.
[55] ZHANG Z T,WEN G H,LIAO J L,et al. Observations of crystallization in mold slags with varying Al2O3/SiO2 ratio[J]. Steel Research International,2010,81(7):516-528.
[56] WANG Z,SOHN I. Effect of the Al2O3/SiO2 mass ratio on the crystallization behavior of CaO-SiO2-MgO-Al2O3 slags using confocal laser scanning microscopy[J]. Ceramics International,2018,44(16):19268-19277.
[57] YAN X,WANG X,WANG S,et al. Influence of Al2O3/SiO2 and BaO/Al2O3 ratios on rheological and crystallization behavior of CaO-BaO-Al2O3-based mold slags[J]. Iron and Steel Institute of Japan International,2022,62(6):1116-1125.
[58] QIU G X,MIAO D J,WEI X L,et al.Effect of MgO/Al2O3 and CaO/SiO2 on the metallurgical properties of CaO-SiO2-Al2O3-MgO-TiO2 slag[J]. Journal of Non-Crystalline Solids,2022,585:121545.
[59] ZHU X,DING B,WANG H,et al.Phase evolution of blast furnace slags with variation in the binary basicity in a variable cooling process[J]. Fuel,2018,219:132-140.
[60] ZHOU L,WANG W,MA F,et al. A kinetic study of the effect of basicity on the mold fluxes crystallization[J]. Metallurgical and Materials Transactions B,2012,43(2):354-362.
[61] WANG W,DAI S,ZHANG T,et al. Effect of isothermal and cooling rate on crystallization and viscosity of silicomanganese waste slag[J]. Ceramics International,2021,47:13622-13627.
[62] LIN B,WANG H,ZHU X,et al. Crystallization properties of mol-ten blast furnace slag at different cooling rates[J]. Applied Thermal Engineering,2016,96:432-440.
[63] HUFFMAN G P,HUGGINS F E,DUNMYRE G R. Investigation of the high-temperature behaviour of coal ash in reducing and oxidizing atmospheres[J]. Fuel,1981,60(7):585-597.
[64] HURST H J,NOVAK F,PATTERSON J H. Viscosity measurements and empirical predictions for fluxed Australian bituminous coal ashes[J]. Fuel,1999,78(15):1831-1840.
[65] ZHENG K,ZHANG Z,YANG F,et al.Molecular dynamics study of the structural properties of calcium aluminosilicate slags with varying Al2O3/SiO2 ratios[J]. ISIJ International,2012,52(3):342-349.
[66] KONG L,BAI J,LI W,et al. The internal and external factor on coal ash slag viscosity at high temperatures,Part 3: Effect of CaO on the pattern of viscosity-temperature curves of slag[J]. Fuel,2016,179:10-16.
[67] SONG W J,TANG L H,ZHU X D,et al.Effect of coal ash composition on ash fusion temperatures[J]. Energy &Fuels,2010,24:182-189.
[68] KONG L,BAI J,BAI Z,et al. Effects of CaCO3 on slag flow properties at high temperatures[J]. Fuel,2013,109:76-85.
[69] SHU Q F,LIU Y. Effects of basicity,MgO and MnO on mineralogical phases of CaO-FeOx-SiO2-P2O5 slag[J]. Ironmaking &Steelmaking,2018,45(4):363-370.
[70] CHEN Y,LI M,WANG S,et al. Effect of MgO on solidification and crystallization properties of ultrahigh-basicity mold flux[J]. Materials Chemistry and Physics,2022,276:125403.
[71] WANG H,DING B,ZHU X,et al.Influence of Al2O3 content on crystallization behaviors of blast furnace slags in directional solidification process[J]. International Journal of Heat and Mass Transfer,2017,113:286-294.
[72] 李刚,潘伟杰,李民,等. Al2O3对超高碱度连铸保护渣理化性能的影响[J]. 工程科学学报,2023,45(2):234-242.
LI Gang,PAN Weijie,LI Min,et al.Effect of Al2O3 on the physical and chemical properties of ultrahigh-basicity continuous casting mold flux[J]. Chinese Journal of Engineering,2023,45(2): 234-242.
[73] 张江. Al2O3含量对CaO-SiO2-Al2O3-CaF2-Na2O保护渣结晶性能的影响[J]. 铸造技术,2011,32(4):511-513.
ZHANG Jiang. Influence of the Al2O3 content on the crystallization properties of CaO-SiO2-Al2O3-CaF2-Na2O mold fluxes[J]. Foundry Technology,2011,32(4):511-513.
[74] WANG S,JIANG Y,GUO Y,et al. Effects of basicity and Al2O3
content on viscosity and crystallization behavior of super-high-alumina slag[J]. Crystals,2022,12(6):851.
[75] ZHANG T,ZHANG H,DAI S,et al.Variation of viscosity and cry-stallization properties of synthetic ferronickel waste slag with Al2O3 content[J]. Ceramics International,2021,47(16):22918-22923.
[76] WANG X,ZHANG Y,CUI L,et al.Effect of SiO2/Al2O3 ratio on crystallization and dielectric properties of barium strontium titanate glass-ceramics[J]. Ferroelectrics,2013,442(1):109-114.
[77] QIN Y L,LV X W,ZHANG J,et al. Determination of optimum blast furnace slag cooling rate for slag recycling in cement manufacture[J]. Ironmaking and Steelmaking,2015,42(5):395-400.
[78] KÖLBL N,MARSCHALL I,HARMUTH H. Single hot thermocouple technique for the characterization of the crystallization behavior of transparent or translucent liquids[J]. Journal of Materials Science,2011,46(19):6248-6254.
[79] ZHU X,DING B,WANG H,et al. Phase evolution of blast fu-rnace slags with variation in the binary basicity in a variable cooling process[J]. Fuel,2018,219:132-140.
[80] CHOI M W,JUNG S M. Crystallization behavior of melted BOF slag during non-isothermal constant cooling process[J]. Journal of Non-Crystalline Solids,2017,468:105-112.
[81] KONG L,BAI J,BAI Z,et al. Improvement of ash flow properties of low-rank coal for entrained flow gasifier[J]. Fuel,2014,120:122-129.
[82] JIANG Y,IDETA K,KIM J,et al. The crystalline and microstructural transformations of two coal ashes and their quenched slags with similar chemical compositions during heat treatment[J]. Journal of Industrial and Engineering Chemistry,2015,22:110-119.
[83] SHEN Z,LI R,LIANG Q,et al.Effect of cooling process on the ge-neration and growth of crystals in coal slag[J]. Energy &Fuels,2016,30(6):5167-5173.
[84] XUAN W,ZHANG J,XIA D. Crystallization characteristics of a coal slag and influence of crystals on the sharp increase of viscosity[J]. Fuel,2016,176:102-109.
[85] XUAN W,WANG Q,ZHANG J,et al.Influence of silica and alumina (SiO2 Al2O3) on crystallization characteristics of synthetic coal slags[J]. Fuel,2017,189:39-45.
[86] SCHWITALLA D H,BRONSCH A M,KLINGER M,et al. Analy-sis of solid phase formation and its impact on slag rheology[J]. Fuel,2017,203:932-941.
[87] REN Q Q,ZHANG Y Z,LONG Y,et al.Investigation on the effect of MgO content on the crystallization behavior of synthetic BF slag[J]. Materials and Manufacturing Processes,2018,33(15):1654-1660.
[88] ILYUSHECHKIN A,KONDRATIEV A,HE C,et al.Viscosity of spinel primary phase field slags from Australian brown coals[J]. Energy &Fuels,2020,34(3):3041-3056.
[89] HE C,ILYUSHECHKIN A,HLA S S. In situ measurements of solid-phase kinetic growth during slag isothermal crystallisation[J]. Journal of Crystal Growth,2020,534:125509.
[90] SCHUPSKY J P,SAAR O,WU G,et al.Viscosity and crystal morphology data of anorthite bearing synthetic coal slag systems[J]. Fuel,2020,280:118663.
[91] REN Q,ZHANG Y,LONG Y,et al.Crystallisation behaviour of blast furnace slag modified by adding fly ash[J].Ceramics International,2018,44(10):11628-11634.
[92] SCHWITALLA D H,BRONSCH A M,KLINGER M,et al.Analy-sis of solid phase formation and its impact on slag rheology[J]. Fuel,2017,203:932-941.
[93] LI J,MOU Q,ZENG Q,et al. Experimental study on precipitation behavior of spinels in stainless steel-making slag under heating treatment[J]. Process Modeling in Pyrometallurgical Engineering,2019,7(8): 487.
[94] XUAN W,ZHANG J,XIA D. Crystallization characteristics of a coal slag and influence of crystals on the sharp increase of viscosity[J]. Fuel,2016,176:102-109.
[95] ZENG Q,LI J,MOU Q,et al.Effect of FeO on spinel crystallizati-on and chromium stability in stainless steel-making slag[J]. Journal of Metals,2019,71(7):2331-2337.
[96] NOWOK J W. Viscosity and phase transformation in coal ash slags near and below the temperature of critical viscosity[J]. Energy and Fuels,1994,8(6):1324-1336.
[97] 孔令学,白进,李文,等. 氧化钙含量对灰渣流体性质影响的研究[J]. 燃料化学学报,2011,39(6):407-412.
KONG Lingxue,BAI Jin,LI Wen,et al. Effect of lime addition on slag fluidity of coal ash[J]. Journal of Fuel Chemistry and Technology,2011,39(6):407-412.
[98] 白进,孔令学,李怀柱,等 . 山西典型无烟煤灰流动性的调控[J]. 燃料化学学报,2013,41(7):805-813.
KONG Lingxue,BAI Jin,LI Wen,et al. Effect of lime addition on slag fluidity of coal ash[J]. Journal of Fuel Chemistry and Technology,2013,41(7):805-813.
[99] KONDRATIEV A,ILYUSHECHKIN A. Flow behaviour of crystallising coal ash slags: Shear viscosity,non-Newtonian flow and temperature of critical viscosity[J]. Fuel,2018,224:783-800.
[100] JEFFREY D J,ACRIVOS A. The rheological properties of suspensions of rigid particles[J]. AIChE Journal,1976,22(3):417-432.
[101] JINESCU V V. Rheology of suspensions[J]. International Journ-al of Chemical Engineering,1974,14(3):397-420.
[102] 沈中杰,郭晓镭,梁钦锋,等. 基于晶体生长及形貌的煤灰渣黏温模型[J]. 化工学报,2021,72(10):5040-5052.
SHEN Zhongjie,GUO Xiaolei,LIANG Qinfeng,et al.Development of coal ash/slag viscosity-temperature model based on crystal growth and morphologies[J]. CIESC Journal,2021,72(10): 5040-5052.
[103] HOY H R,ROBERTS A G,WILKINS D M. Behavior of mineral matter in slagging gasification processes[J]. 1965:3-27.
[104] 宋文佳. 高温煤气化炉中煤灰熔融、流动和流变行为特性研究[D]. 上海:华东理工大学; 2011:165-168.
[105] KONG L,BAI J,BAI Z,et al.Effects of CaCO3 on slag flow properties at high temperatures[J]. Fuel,2013,109:76-85.
[106] HE C,ILYUSHECHKIN A,HLA S S. In situ measurements of solid-phase kinetic growth during slag isothermal crystallisation[J]. Journal of Crystal Growth,2020,534:125509.
[107] HIRAI M,TAKEBAYASHI K,YOSHIKAWA Y,et al.Apparent viscosity of Al-10mass% Cu semi-solid alloys[J]. Iron and Steel Institute of Japan International,1993,33(3):405-412.
[108] WRIGHT S,ZHANG L,SUN S,et al.Viscosities of calcium ferrite slags and calcium alumino-silicate slags containing spinel particles[J]. Journal of Non-Crystalline Solids,2001,282(1):15-23.
[109] WRIGHT S,ZHANG L,SUN S,et al. Viscosity of a CaO-MgO-Al2O3-SiO2 melt containing spinel particles at 1 646 K[J]. Metallurgical &Materials Transactions B,2000,31(1):97-104.
[110] LIU Z,CHEN L,BLANPAIN B,et al.Effect of crystallization on the abrupt viscosity increase during the slag cooling process[J]. Iron and Steel Institute of Japan International,2018,58(11):1972-1978.
[111] MOITRA P,GONNERMANN H. Effects of crystal shape-and size-modality on magma rheology[J]. Geochemistry Geophysics Geosystems,2015,16(1): 1-26.
[112] ILYUSHECHKIN A,KONDRATIEV A. Viscosity of slags with solids: The effect of solids morphology and concentration[J]. Journal of Rheology,2019,63(5):719-733.
[113] BRUIJN H D. The viscosity of suspensions of spherical particles[J]. Recueil des Travaux Chimiques des Pays-Bas,2010,61(12):863-874.
[114] ILYUSHECHKIN A Y,HLA S S,ROBERTS D G,et al.The eff-ect of solids and phase compositions on viscosity behaviour and TCV of slags from Australian bituminous coals[J]. Journal of Non-Crystalline Solids,2011,357(3):893-902.
[115] SAAR MO,MANGA M,CASHMAN K V,et al.Numerical mod-els of the onset of yield strength in crystal-melt suspensions[J]. Earth and Planetary Science Letters,2001,187(3):367-379.
[116] NGUYEN Q D. A new rheometer for direct measurement of the flow properties of coal ash at high temperatures[J]. Fuel,2002,81(4): 397-404.
[117] SONG W,TANG L,ZHU Z,et al. Rheological evolution and cr-
ystallization response of molten coal ash slag at high temperatures[J]. AIChE Journal,2013,59(8): 2726-2742.
[118] LIU Z,ANNELIES M,BART B,et al. Rheological transitions of the solid-bearing slag during cooling process[J]. Metallurgical &
Materials Transactions B,2018,49(5): 2649-2657.
[119] SEEBOLD S,WU G,MÜLLER M. The influence of crystallizat-ion on the flow of coal ash-slags[J]. Fuel,2017,187:376-387.
Advances in crystallization behavior of non-Newtonian slags during cooling process