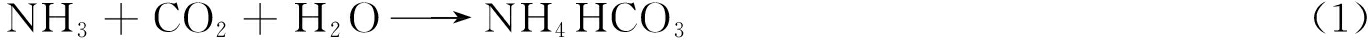低阶煤与聚丙烯共热解协同效应分析
0 引 言
新疆地区煤炭种类丰富,以有高挥发分的低阶煤为主,尤其是哈密地区煤挥发分普遍高,甚至超过50%(干燥无灰基),适宜通过热解提取油气组分,进一步转化为高附加值产品[1],半焦是燃料、气化原料及碳材料等[2],此分级分质利用模式是双碳背景下煤高效清洁转化的重要途径。而热解焦油中重质组分含量高、黏度大、易带尘[3],给分离带来困难。
由于煤热解遵从自由基反应机理[4],要提高焦油产率/品质,引入活性富氢组分来稳定煤热解自由基[5,7],或通过富氢物质与煤共热解强化供氢过程[8-10]实现。与煤相比,通用塑料H/C原子比高,是理想供氢原料[11,13]。WU等[14]发现平朔煤与30%聚苯乙烯(PS)混合热解时,甲基苯酚和三甲基萘的相对含量比理论计算值分别减少33%和80%,但甲苯提高14.8倍,因为加入PS有利于苯酚与烯烃反应生成芳烃,增强苯酚脱水反应,促进多环芳烃分解产生苯和单环芳烃。此外,焦油中苯乙烯提高3.41倍,归因于共热解过程促进了聚苯乙烯主链上Cβ—Car键的断裂。当高密度聚乙烯(HDPE)与褐煤(1∶1)在500 ℃热解时,气体及焦油产率分别比理论值高8.59%和21.94%,而焦炭产率则减少30.53%,因为HDPE热解产生的·H等自由基可稳定煤热解自由基形成挥发物,抑制焦炭生成;此外,焦油组分中烃类约提高8倍,这与HDPE自身的结构特征有关[15]。虽然塑料是外在氢源,对焦油生成有利,但其加入过多会延长挥发物停留时间,加剧二次裂解[16]。聚苯乙烯因含芳烃结构,更易分解,与煤共热解的活化能比聚丙烯和聚乙烯低约40 kJ/mol,此外,聚苯乙烯在热解过程中抑制了煤的流动性,引起氧之间的交联反应,导致CO2产率增加[17]。ZHANG等[18]发现低阶煤与高密度聚乙烯(HDPE)分层放置时,焦油H/C原子比煤单独热解高0.19,因为HDPE产生的自由基是氢供体,促进煤中芳香族化合物的裂解。此外,焦油重质组分比二者混合时约低10%,分析认为分层放置诱发了半焦基体内催化反应,促进大分子挥发物裂解。
综上,塑料热解挥发物不仅与煤热解挥发物产生相互作用,还吸附于半焦表面或孔道中,在活性位点/矿物质作用下进一步反应,其效果与二者接触方式密切相关。为揭示该作用机制,基于聚丙烯热解过程中可产生丰富活性小分子自由基的特点,选择聚丙烯于煤上方分层放置,探究不同比例淖毛湖煤(NMH)与聚丙烯(PP)共热解过程中的失重,基于协同效应分析获取促进挥发物释出效果最显著的煤塑比,利用TG-FTIR-GC/MS在线监测该比例下热解产物逸出特征,揭示二者共热解过程中挥发物相互作用机制。
1 试 验
1.1 试验原料
试验原料为新疆淖毛湖煤(NMH)和东莞市樟木头华创塑胶原料商行提供的聚丙烯(PP)。将二者粉碎、筛分至0.15~0.18 mm,于105 ℃干燥3 h后取出密封保存。工业分析及元素分析见表1。NMH的H/C高达0.99,PP的H/C比为1.97,约为NMH的2倍。
表1 原料的工业分析与元素分析
Table 1 Proximate analysis and ultimate analysis of raw materials

注:*差减法。
当NMH与PP按质量比9∶1、8∶2、7∶3和6∶4分层放置时,记为9NMH-1PP、8NMH-2PP、7NMH-3PP和6NMH-4PP。
1.2 试验方法
采用TG-MS联用仪(日立,日本)在线监测PP热失重过程中挥发性物质逸出行为,样品质量(10±0.2) mg,以100 mL/min的高纯氮气为载气,10 ℃/min升温速率由室温升至终温800 ℃。
通过珀金埃尔默的TG-FTIR-GC/MS分析NMH与PP共热解过程中气体与焦油组分逸出特征。该仪器由热重分析仪(TGA8000)、傅里叶红外光谱仪(Frontier)和GC/MS(Clarus680/SQ8T)组成。先将不同比例NMH和PP分层置于坩埚中(总质量(10±0.2) mg),下层为PP,中间用薄层石英棉隔开,使PP热解挥发物可随载气与NMH相互作用;接着以100 mL/min高纯N2吹扫整个热解体系20 min,确保空气被排尽,最后以10 ℃/min升温至终温800 ℃,测得NMH-PP失重曲线。采用在线联用监测时,将TGA温度升至40 ℃时热解气与载气一同泵入红外光谱仪中实时检测,泵速30 mL/min。根据失重特征,在选定数据采集温度下通过GC/MS分析产物成分。
条件与步骤同上,在TGA8000热重分析仪中探究不同升温速率(β=10、20和30 ℃/min)下NMH和PP及8NMH-2PP的失重行为。
1.3 协同效应分析
为量化NMH和PP共热解时相互作用程度[19],从热失重过程中质量变化角度分析二者协同效应,见式(1)和式(2):
Δw=wexp-wcal,
(1)
wcal=x1w1 x2w2,
(2)
式中,Δw为协同值;wexp、wcal分别为共热解样品的试验值和理论加和值,%;x1、x2分别为共热解中NMH和PP质量分数,%;w1、w2分别为NMH和PP单独热解的试验失质量,%。
根据Lambert-Beer定律,相同试验条件下,红外光谱测定的官能团吸收强度与物质的含量成正比,故气体产物FTIR光谱试验值和计算值[20]可用于描述相互作用,如式(3)所示:
ABScal=x1ABSexp x2ABSexp,
(3)
式中,ABScal、ABSexp分别为FTIR光谱的试验值与理论加和值。
1.4 动力学方法
采用Flynne-Walle-Ozawa(FWO)等转化率法计算NMH与PP共热解活化能[21]。根据Arrhenius方程,反应速率式(4)及变形式式(5)如下:

(4)

(5)

(6)
式中,α为原料转化率;t为时间;k(T)为反应速率常数;f(α)为反应模型函数;A为指前因子,min-1;E为表观活化能,kJ/mol;R为通用气体常数,8.314 J/(mol·K);T为热力学温度;β为升温速率,℃/min;m0、mt、m∞分别为初始、t时刻和最终时刻的样品质量,g。

(7)

(8)
式中,g(α)为f(α)积分;T0、T分别为初始温度和反应温度,K;P(u)为温度函数的指数积分形式。
基于式(8),FWO方程表达式如下:

(9)
其中,活化能E由ln β与1/T拟合求得。
2 结果与讨论
2.1 淖毛湖煤与聚丙烯的热失重特性与协同效应
图1给出了氮气气氛下NMH与PP的热失重曲线及PP热解挥发物TG-MS曲线。由图1(a)可知,NMH主要热解区间为340~600 ℃。PP从370 ℃开始分解,当温度高于500 ℃时几乎完全分解(图1(b)),说明二者主要热解温度区间存在较大范围重叠,为PP热解产物中富氢组分及活性自由基在共热解时参与到煤热解过程提供可能[22]。

图1 NMH和PP的TG-DTG曲线及PP热解挥发物的TG-MS曲线
Fig.1 TG-DTG curves of NMH and PPand TG-MS curves of volatiles during PP pyrolysis
由热解气体逸出曲线(图1(c))可知,PP热解过程中H2和·CH3初始释出温度略低,为394和377 ℃,于506和554 ℃释放完全。CH4和C2H6生成温度较高,分别升至407和398 ℃,释放完全的温度降至498和515 ℃,因为PP中含大量—CH3侧链,受热过程中易断裂,同时产生·H和·CH3,前者相互结合成H2,后者为C2H6。CH4由聚合物链随机断裂或二次反应生成[23]。综上,共热解过程中PP有稳定煤热解挥发物潜能。
为阐明NMH与PP共热解过程中挥发分的释放,探究2种物料不同比例下的热失重。图2(a)给出失质量的试验值与理论计算值,差异大,说明NMH与PP共热解过程存在相互作用。40~536 ℃,实际失质量小于理论失质量,说明PP抑制挥发物生成,该效应于约500 ℃最强(图2(b))。因为PP热解过程中随温度升高会软化到塑性状态,使挥发物释放减缓[24],且该抑制效应随PP增加而增强(图2(b)),这与PP在此过程中膨胀有关[25]。温度继续升高,PP开始分解[26],短时期挥发分大量释放,产生的·CH3和·H等自由基(图1(c))可以抑制NMH热解生成的分子碎片相互结合[27],因而500~536 ℃区间抑制作用减弱,促进作用增强。温度升至536 ℃以上,实际失质量大于计算值,因为PP释放的大量挥发物促使煤热解半焦形成多孔结构,暴露出更多活性位点,利于被吸附的PP热解挥发物中大分子碎片裂解[28],产生的自由基亦可作为氢供体,促进煤中化学键断裂[17],NMH与PP质量比8∶2(8NMH-2PPexp)时,在552 ℃时协同效应最显著,协同值Δw为4.23%。

图2 NMH-PP的TG曲线和共热解协同效应
Fig.2 TG curves and co-pyrolysis synergistic effect of NMH-PP
2.2 热解挥发产物释出规律
2.2.1 气体逸出行为
采用In-situ FTIR分析NMH和PP单独热解及二者最优比例下(8NMH-2PPexp)共热解中气体释出规律,结果如图3所示。热解气体产物和官能团对应的FTIR波数见表2。可知,产物种类随温度升高而增加[29]。8NMH-2PPexp产物峰与NMH和PP单独热解相似,但强度不同,说明共热解存在相互作用[30],CH4及1 460 ~1 510 cm-1烃类峰强度明显增加,这可能与共热解过程中PP自身热解产物释放有关[31]。

图3 NMH、PP和8NMH-2PPexp热解产物的3D-FTIR谱图
Fig.3 3D-FTIR spectra of pyrolysis products of NMH,PP and 8NMH-2PPexp
表2 热解气体产物和官能团对应的FTIR波数
Table 2 FTlR wavenumbers of gas species and functional groups from pyrolysis products

与NMH相比,整个热解过程8NMH-2PPexp挥发物释出温度区间更集中,CO2与H2O变化明显,因为热解前期PP软化作用阻碍NMH中小分子释放。共热解挥发物释放结束温度比PP单独热解高,因为PP与NMH间相互作用稳定NMH挥发物释出的同时也延缓PP自身分解[32]。结合TG曲线(图1(a)、(b))可知,室温至约400 ℃时NMH失重主要为吸附分子如CO2、H2O等的脱除和易分解产物的逸出,PP此时基本无产物特征峰,这与其C—C、C—H和C![]() C键分解温度高有关[33]。为进一步阐释热解及共热解过程反应机制,分析4种主要释出小分子气体在热解过程中吸光度计算值与试验值,如图4所示。
C键分解温度高有关[33]。为进一步阐释热解及共热解过程反应机制,分析4种主要释出小分子气体在热解过程中吸光度计算值与试验值,如图4所示。

图4 主要热解气体的2D-FTIR谱图
Fig.4 2D-FTIR spectra of the main pyrolysis gases
由图4(a)可知,CO2有2个主要逸出峰。低温处源自煤中吸附CO2、脂肪键、醚键和羰基等的断裂,高温处归因于煤中矿物质分解[34],共热解峰强度试验值低于理论值,可能因为PP裂解时生成的·CH3、·H等活性组分改变NMH挥发物中C和O的释放形式,抑制二者结合形成CO2[29]。CO生成分为2阶段(图4(b)):温度≤579 ℃,主要来自甲氧基、醛和羧基的分解;温度≥600 ℃,源于羟基、醚和含氧杂环的断裂[35]。第一温度区间共热解实际释放量明显高于理论值,因为PP热解产生大量富氢组分促进NMH中甲氧基、醛等含氧基团断键[36]。
由图4(c)可知,PP单独热解释放大量CH4,但共热解试验峰强度(8NMH-2PPexp)远低于理论值(8NMH-2PPcal),表现为强抑制,因为本应形成CH4(·CHx ·H![]() CH4)[37]的·CH3、·H等参与稳定煤热解挥发物过程。H2O有2个逸出峰(图4(d)),约100 ℃峰强度弱,与煤中较低自由水含量有关[38]。400~600 ℃,与CO逸出峰相似,H2O峰也明显增强,表现为显著促进作用,因为PP热解挥发物中富氢组分与NMH中甲氧基、羟基及羧基等官能团发生加氢反应,使其脱除[39]。
CH4)[37]的·CH3、·H等参与稳定煤热解挥发物过程。H2O有2个逸出峰(图4(d)),约100 ℃峰强度弱,与煤中较低自由水含量有关[38]。400~600 ℃,与CO逸出峰相似,H2O峰也明显增强,表现为显著促进作用,因为PP热解挥发物中富氢组分与NMH中甲氧基、羟基及羧基等官能团发生加氢反应,使其脱除[39]。
2.2.2 挥发物组成
基于TGA分析,8NMH-2PPexp在552 ℃促进作用最佳(图2(b)),故采用气质联用仪识别样品在此温度下的热解挥发物(图5(a)),对其归类(图5(b)),分析PP与NMH共热解时协同效应Δw(图5(c))。NMH热解产物峰多,PP的相对少,但强度高,8NMH-2PPexp峰数量与PP的更接近。对于产物组成,NMH的以烷烃和CO2为主,相对含量分别为34.67%和14.84%(图5(b)),醚、醇、醛类含氧组分高于芳香化合物,这源于煤大分子结构中脂肪族及C—O桥键、醚键等含氧化合物裂解。PP产物主要为烯烃及烷烃类,碳数范围在C18~C35,出峰时间晚(图5(a)),与其自身含大量支链的结构特征和随机断链热解机制有关[40]。8NMH-2PPexp中CO2、烯烃及烷烃等产物强度与PP和NMH单独热解时差异大,且新生成2,3,3-三甲基-1-己烯等烯烃及3,7,11-三甲基-1-十二醇等醇类特征峰(图5(a))。保留时间≥45 min时出峰少,说明添加PP抑制重质组分生成。

图5 各样品热解产物的组成和协同效应分析
Fig.5 Composition and synergistic effect analysis of pyrolysis products for each sample
与8NMH-2PPcal相比,共热解(8NMH-2PPexp)产物中烯烃和醇类相对含量显著增加(图5(b)),前者增幅30.58%(图5(c)),这与残留PP大分子碎片经二次反应与NMH热解挥发物相互作用形成含不同甲基侧链的烯烃有关。PP形成的短链分子与煤中羟基等含氧物质结合生成醇类[41],导致醇类增加16.18%。CO2和烷烃类分别减少8.89%和14.43%,酚及其他含氧等物质分别减少6.79%和7.12%。经分析,烷烃降低因为PP产生的自由基阻碍了NMH热解生成·CH3和·CH2等反应[42],PP热解挥发物也诱发挥发物与煤热解半焦的反应[43]。CO2减少说明PP和NMH相互作用影响C![]() O转化[43],酚和醛类含量降低与PP热解挥发物中富氢组分促使NMH中甲氧基、羟基及羧基等含氧物质分解有关,与图4中CO和H2O红外变化一致[45]。综上,PP和NMH共热解过程中存在明显协同效应。
O转化[43],酚和醛类含量降低与PP热解挥发物中富氢组分促使NMH中甲氧基、羟基及羧基等含氧物质分解有关,与图4中CO和H2O红外变化一致[45]。综上,PP和NMH共热解过程中存在明显协同效应。
2.3 动力学分析
为进一步揭示共热解过程中相互作用,用FWO计算NMH、PP及8NMH-2PPexp不同转化率下动力学参数,见表3。R2均≥0.96,拟合效果好。NMH和PP单独热解,α=0.1~0.5时,二者活化能均升高,说明转化初期物料需吸收高能量断裂化学键,生成新物质[46]。α>0.5时,NMH活化能逐渐下降,PP的基本不变,归因于煤中挥发性物质随热解反应程度加深而减少和PP基本分解完全[22]。经对比,α<0.7时,共热解试验活化能值(8NMH-2PPexp)均低于理论值(8NMH-2PPcal),α>0.7则相反。因为转化前期主要为挥发物-挥发物之间的反应,PP是氢供体可及时稳定NMH热解自由基,使反应活化能降低;共热解后期,被吸附在半焦内的PP挥发物诱发的挥发物-半焦反应占主导,挥发物逸出需高能量。总体而言,8NMH-2PP平均活化能试验值比理论值低6.8 kJ/mol,说明PP与NMH之间相互作用降低了共热解活化能,促进挥发物释放。
表3 采用FWO计算各样品不同转化率下动力学参数
Table 3 Kinetic parameters of each sample obtained from FWO

3 结 论
1)NMH和PP以不同质量比分层放置时,挥发物释出作用规律存在差异,质量比8∶2(8NMH-2PPexp)时,促进效应最大,Δw为4.23%。
2)共热解过程中PP和NMH间相互作用促使氧元素以CO与H2O形式释出。烯烃及醇类物质比理论值分别增加30.58%和16.18%,CO2和烷烃类分别降低8.89%和14.43%,共热解产物较单独热解呈现轻质化趋势。
3)添加PP促进了NMH挥发物释放,共热解平均活化能试验值(8NMH-2PPexp)比理论值低(8NMH-2PPcal)6.8 kJ/mol。
[1] ZHANG D, LIU P, LU X, et al.Upgrading of low rank coal by hydrothermal treatment: Coal tar yield during pyrolysis[J]. Fuel Processing Technology, 2016, 141: 117-122.
[2] JIANG T, LIU S, ALMUTAWA F, et al. Comprehensive reuse of pyrolysis chars from coals for fabrication of highly insulating building materials[J]. Journal of Cleaner Production, 2019, 222: 424-435.
[3] HE W, YIN G, ZHAO Y, et al. Interactions between free radicals during co-pyrolysis of lignite andbiomass[J]. Fuel, 2021, 302: 121098.
[4] HE X, ZHU H, HUO Y, et al.Study on the formation mechanism of the pyrolysis products of lignite at different temperatures based on ReaxFF-MD[J].ACS Omega 2021, 6 (51): 35572-35583.
[5] NIU B, NIU M, ZHANG J, et al. Novel insight into the mechan-ism of coal hydropyrolysis using deuterium tracer method[J]. Fuel, 2022, 321: 124109.
[6] WANG Z, WEI B, LU J, et al. In-situ catalytic upgrading of tar from integrated process of coal pyrolysis with steam reforming of methane over carbon based Ni catalyst[J]. Journal of Fuel Chemistry, 2022, 50(2):129-142.
[7] WU Y, WANG G, ZHU J. Insight into synergistic effect of co-pyrolysis of low-rank coal and waste polyethylene with or without additives using rapid infraredheating[J]. Journal of the Energy Institute, 2022,102:384-394.
[8] YAO Z, KANG K, CONG H, et al. Demonstration and multi-perspective analysis of industrial-scale co-pyrolysis of biomass, waste agricultural film, and bituminous coal[J]. Journal of Cleaner Production, 2021, 290: 125819.
[9] SIDDIQI H, MISHRA A, MAITI P, et al. In-situ and ex-situ co-pyrolysis studies of waste biomass with spent motor oil: Elucidating the role of physical inhibition and mixing ratio to enhance fuelquality[J]. Bioresource Technology, 2022: 358: 127364.
[10] LI J, ZHU J, HU H, et al.Co-pyrolysis of Baiyinhua lignite and pine in an infrared-heated fixed bed to improve tar yield[J]. Fuel, 2020, 272: 117739.
[11] ZHOU L, LUO T, HUANG Q. Co-pyrolysis characteristics and kinetics of coal and plasticblends[J]. Energy Conversion and Management, 2009, 50(3): 705-710.
[12] WENY, LIU S, FU S, et al. Insight into influence of process parameters on co-pyrolysis interaction between Yulin coal and waste tire via rapid infrared heating[J]. Fuel, 2023, 337: 127161.
[13] ISHAQ M, AHMAD I, SHAKIRULLAH M, et al. Pyrolysis of some whole plastics and plastics-coalmixtures[J]. Energy conversion and management, 2006, 47(18/19): 3216-3223.
[14] WU Y, ZHU J, ZHAO S, et al.Co-pyrolysis behaviors of low-rank coal and polystyrene with in-situ pyrolysis time-of-flight mass spectrometry[J]. Fuel, 2021, 119461.
[15] KOJI I, BECHTEL A, ALEKSI N, et al. Study of the synergetic effect of co-pyrolysis of lignite and high-density polyethylene aiming to improve utilization of low-rankcoal[J]. Polymers, 2021, 13(5): 759.
[16] QIAN C, ZHOU M, WEI J, et al.Pyrolysis and co-pyrolysis of lignite and plastic[J]. International Journal of Mining Science and Technology, 2014, 24(1): 137-141.
[17] MELENDI E S, ALVAREZ R, DIEZ M A, et al. Coal and plastic waste co-pyrolysis by thermal analysis-mass spectrometry[J]. Fuel Processing Technology, 2015, 137: 351-358.
[18] ZHANG T, YUCHI W, BAI Z, et al.Insight into the chargi-ng methods effects during clean recycling of plastic by co-pyrolysis with low-rank coal[J]. Journal of Cleaner Production, 2022, 333: 130168.
[19] WU Z, WANG S, ZHAO J, et al. Synergistic effect on thermal behavior during co-pyrolysis oflignocellulosic biomass model components blend with bituminous coal[J]. Bioresource Technology, 2014, 169: 220-228.
[20] NI Z, BI H, JIANG C, et al.Investigation of the co-pyrolysis of coal slime and coffee industry residue based on machine learning methods and TG-FTIR: Synergistic effect, kinetics and thermodynamic[J]. Fuel, 2021, 305: 121527.
[21] LY X, LIU H, HUANG Y, et al.Synergistic effects on co-pyrolysis of low-temperature hydrothermally pretreated high-protein microalgae and polypropylene[J]. Energy Conversion and Management, 2021, 229: 113772.
[22] SHARMA S, GHOSHAL A K. Study of kinetics of co-pyrolysis of coal and waste LDPE blends under argonatmosphere[J]. Fuel, 2010, 89(12): 3943-3951.
[23] SCHEIRS J, KAMINSKY W. Feedstock recycling and pyrolysis of waste plastics: converting waste plastics into diesel and other fuels[D].Chichester:Johawilley &Suns. Inc, 2006.
[24] LI S, CHEN X, LIU A, et al. Co-pyrolysis characteristic of biomass and bituminous coal[J].Bioresource Technology, 2015, 179: 414-420.
[25] GUO D L, YUAN H Y, YIN X L,et al.. Effects of chemical form of sodium on the product characteristics of alkali lignin pyrolysis[J]. Bioresource Technology, 2014, 152: 147-153.
[26] BOCKHORN H, HORNUNG A, HORNUNG U, et al. Kinetic study on the thermal degradation of polypropylene andpolyethylene[J]. Journal of Analytical and Applied Pyrolysis, 1999, 48(2): 93-109.
[27] XU X, ZHAO B, SUN M, et al.Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis[J]. Waste Management, 2017, 62: 91-100.
[28] HAN J, WANG X, YUE J, et al.Catalytic upgrading of coal pyrolysis tar over char-based catalysts[J]. Fuel Processing Technology, 2014, 122: 98-106.
[29] LIN, CHEN, ZHAO,SHENG, et al. Co-pyrolysis of chlorella vulgaris and kitchen waste with different additives using TG-FTIR and Py-GC/MS[J]. Energy conversion and management, 2018, 177: 582-591.
[30] FAEOOQ M Z, ZEESHAN M, IQBAL S, et al. Influence of wa-ste tire addition on wheat straw pyrolysis yield and oilquality[J]. Energy, 2018, 144: 200-206.
[31] KAI X, YANG T, SHEN S, et al. TG-FTIR-MS study of synergistic effects during co-pyrolysis of corn stalk and high-density polyethylene (HDPE)[J]. Energy Conversion and Management, 2019, 181: 202-213.
[32] WU X, WUY, WU K, et al. Study on pyrolytic kinetics and behavior: The co-pyrolysis of microalgae and polypropylene[J]. Bioresource technology, 2015, 192: 522-528.
[33] LI J, YAO X, XU K. A comprehensive model integrating BP neural network and RSM for the prediction and optimization of syngasquality[J]. Biomass and Bioenergy, 2021, 155: 106278.
[34] GENG W, NAKAJIMA T, TAKANASHI H, et al. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry[J]. Fuel, 2009, 88(1):139-144.
[35] ZHOU W, LIN Q, WANG C, et al.Co-Pyrolysis behavior of coal slime and Chinese medicine residue by TG-FTIR-MS with principal component analysis and artificial neural network model[J]. Combustion Science and Technology, 2023,195(8):1840-1872.
[36] 赵淑蘅, 蒋剑春, 孙云娟,等. 稻壳与褐煤共热解过程的TG-FTIR分析[J]. 煤炭转化, 2013,36(1): 19-23.
ZHAO Shuheng, JIANG Jianchun, SUN Yunjuan, et al. TG-FTIR analysis of co pyrolysis process of rice hull and lignite[J]. Coal Conversion, 2013,36(1): 19-23.
[37] DING Y,EZEKOYE O A,LU S,et al. Thermal degradation of beech wood withthermogravimetry/Fourier transform infrared analysis[J]. Energy Conversion &Management, 2016, 120: 370-377.
[38] TIAN L, SHEN B, XU H,et al. Thermal behavior of waste tea pyrolysis by TG-FTIR analysis[J]. Energy, 2016, 103:533-542.
[39] 芦海云, 陈爱国, 郜丽娟, 等. 热重-红外联用研究上湾煤中低温热解行为[J]. 煤炭转化, 2015, 38(3): 32-35.
LU Haiyun, CHEN Aiguo, GAO Lijuan, et al. Thermogravimetric and infrared study on the pyrolysis behavior of shangwan coal at medium and low temperatures[J]. Coal Conversion,2015,38(3):32-35.
[40] 余建平. 基于TGA-FTIR-GC/MS联机技术的环境中微塑料定量分析研究[D]. 杭州: 浙江工业大学, 2019.
[41] 刘世奇, 张素平, 于泰莅, 等. 生物质与塑料共热解协同作用研究[J]. 林产化学与工业, 2019, 39(3):34-42.
LIU Shiqi, ZHANG Suping, YU Taili, et al.Study on the synergistic effect of biomass and plastic co pyrolysis[J]. Chemistry and Industry of Forest Products, 2019, 39(3):34-42.
[42] LI S, LI J, CHEN H. Understanding the release behavior of biomass model components and coal in the co-pyrolysis process[J]. Journal of the Energy Institute, 2022, 101: 122-130.
[43] TIAN F J, ZHANG S, HAYASHI J I, et al. Formation of NOx precursors during the pyrolysis of coal and biomass. Part X: Effects of volatile-char interactions on the conversion of coal-N during the gasification of a Victorian brown coal in O2 and steam at 800 ℃[J]. Fuel, 2010, 89(5): 1035-1040.
[44] LIU X, BURRA K G, WANG Z, et al. On deconvolution for understanding synergistic effects in co-pyrolysis of pinewood and polypropylene[J]. Applied Energy, 2020, 279: 115811.
[45] ZHANG M, RESENDE F L, MOUTSOGLOU A, et al. Pyrolysis of lignin extracted from prairiecordgrass, aspen, and Kraft lignin by Py-GC/MS and TGA/FTIR[J]. Journal of Analytical and Applied Pyrolysis, 2012, 98: 65-71.
[46] MISHRA R K, MOHANTY K. Kinetic analysis and pyrolysisbehaviour of waste biomass towards its bioenergy potential[J]. Bioresource Technology, 2020, 311: 123480.
Synergetic effect during co-pyrolysis of low-rank coal and polypropylene

移动阅读