CO2和H2O气化反应对富氧气氛煤热解和焦炭燃烧影响进展
0 引 言
我国是以原煤为主要化石能源的能源消耗大国,能源消耗中原煤占比60%以上。其中,火力发电是煤炭消耗的主要原因之一,同时也是温室气体的主要排放来源[1]。习近平主席曾在第75届联合国大会上宣布,我国将对CO2排放采取强有力的治理举措,争取在2030年前实现碳达峰,2060年前实现碳中和[2]。因此降低燃煤电站CO2排放对于我国未来环境和能源发展尤为重要。
富氧燃烧技术最初由ABRAHAM等[3]于1982年提出并应用于石油开采,目的是降低CO2封存成本,提高石油产量。经过近几十年发展,富氧燃烧技术被认为是最有潜力规模化应用的燃煤电站CO2减排技术之一[4]。燃煤电站常压富氧燃烧技术系统使用空气分离系统将空气中氧气富集,并与循环烟气混和代替空气作为氧化剂送入炉膛燃烧,系统尾部烟气中CO2体积分数达90%左右,具有CO2捕集相对成本较低、可改造存量机、易规模化等优势。
2000年左右,美国麻省理工学院FASSBENDER[5]在常压富氧燃烧技术基础上首次提出加压富氧燃烧技术,该系统中的氧气分离、煤粉燃烧和CO2压缩均在高压下进行,降低了因常压富氧燃烧系统压力波动造成的能量损失,同时可通过回收烟气中部分汽化潜热进一步提高机组效率。
常规空气燃烧中,烟气中最主要的气体成分为N2(摩尔分数约70%),但富氧燃烧系统中,烟气主要组分为CO2(摩尔分数60%~70%)和H2O(摩尔分数25%~35%)。由于CO2、H2O与N2在物理性质上的明显差异以及碳与CO2、H2O的气化反应作用,煤粉在富氧气氛中的燃烧过程与常规空气燃烧有明显区别。煤粉在O2/CO2/H2O气氛中的反应特性是富氧燃烧技术的基础,掌握CO2和H2O对煤热解和焦炭燃烧的影响,对解决富氧燃烧发电系统的现实性问题(如燃烧器和锅炉的设计和优化)具有重要的理论意义和实用价值。
1 气体物理性质对煤热解和焦炭燃烧的影响
1.1 气体物理性质计算及分析
为明确不同气体之间物理性质差异,基于热力学模型PR-BM[6]计算了CO2、H2O和N2单组分气体与O2/N2、O2/CO2、O2/CO2/H2O多组分气体的密度、比热容、导热系数和氧气扩散系数,具体见表1。由表1可知,常压下单组分气体比热容的关系为:CO2>H2O>N2,扩散系数为:H2O>N2>CO2,导热系数为:H2O>CO2≈N2,密度为:CO2>N2>H2O。压力升高后,单组分气体的比热容显著升高,导热系数轻微增加,氧气扩散系数明显降低,密度显著增加,但3种气体物理性质的相对关系不变。在常压下,对于多组分气体,21% O2/79% N2气氛中N2被CO2替换后混合气氛具有更高的比热容和密度、更低的导热系数和氧气体扩散系数。氧气体积分数升高至30%,混合气体的密度、比热容和导热系数略降低,而氧气扩散系数基本恒定。若向O2/CO2气氛中加入H2O,混合气体的密度和比热容有所减小,但导热系数和氧气扩散系数有所增加。1.0 MPa时,所有混合气体的密度和比热容均增大,扩散系数均变小,导热系数基本不变。由以上分析可知,富氧气氛的物理性质与空气气氛差异明显,这可能会对煤热解及焦炭燃烧产生一定影响。
表1 1 273 K、0.1和1.0 MPa条件下气体物性参数
Table 1 Gas physical parameters at 1 273 K, 0.1 and 1.0 MPa

1.2 气体物性对煤热解的影响
煤热解是一种将煤加热至高温,使其分解为各种气体和固体残渣的过程。对于挥发分的析出行为,李林等[7]使用煤粒脱挥发分数学模型研究了富氧条件下CO2/N2对煤粉脱挥发分的影响机制。研究发现,在同样条件下,CO2气氛的脱挥发分时间均小于N2气氛,这是由二者物性差异导致。BORREGO等[8]使用沉降炉(Drop Tube Furnace,DTF)对高挥发性和低挥发性烟煤颗粒进行试验,结果表明,N2被CO2取代时,低挥发性煤和高挥发性煤的挥发性产物均减少。由于CO2比热容高于N2(表1),大量CO2气体导致煤颗粒加热速率降低,从而导致挥发性物质产量较低[9]。对于热解生成焦炭的性质,张洪等[10]利用DTF研究了富氧条件下CO2/N2对煤焦反应性的影响。结果表明,在CO2气氛下制备的煤焦孔隙率、比表面积均大于N2气氛,这是由CO2和N2气体导热性能差异导致。
1.3 气体物性对煤粉和焦炭燃烧的影响
CO2物性对燃烧的影响已得出相对一致的结论,即CO2的高比热容和低氧扩散速率等性质导致煤粉和焦炭在富氧气氛中的燃烧温度和速率均降低[7,11-21]。LEI等[22]利用自制恒温热分析系统(Isothermal Thermal Analysis System,ITAS)对煤粉富氧燃烧特性进行研究。结果表明,温度一定时,空气燃烧煤粉的转化率始终高于富氧燃烧,但随氧气浓度升高,2种气氛的差异逐渐缩小,说明在低氧浓度下气氛的物性差异是导致燃烧差异的主要因素,而较高的氧气浓度削弱了N2与CO2物理性质差异的影响,这与后续CAI等[23]和KOPS等[24]使用DTF的研究结论一致。
关于H2O物性对燃烧的影响,YI等[25]使用管式炉(Tube Furnace,TF)研究了煤粉在O2/CO2/H2O气氛中的燃烧特性,发现随H2O浓度升高,煤粉着火温度增加,并认为这是由于具有较高发射率的H2O强化了辐射散热进而造成颗粒温度降低,这与RIAZ等[18]使用夹带流反应器(Entrained Flow Reactor,EFR)的结论一致。LEI等[22]发现,由于O2/CO2/H2O中气体的导热系数相较O2/CO2气氛更高(表1),在O2/CO2气氛中加入H2O后煤粉燃烧速率升高,燃尽时间缩短。此外,也有学者认为H2O物性对燃烧的影响与其浓度有关。LI等[26]使用平焰燃烧器系统(Flat Flame Burner System,FFBRS)研究了30% O2下CO2/H2O对焦炭燃烧的影响机理,发现CO2/H2O体积比接近1∶1时对焦炭燃烧速率起促进作用,但随H2O比例进一步扩大,其物理性质逐渐占据主导地位,导致燃烧速率降低。
2 CO2和H2O气化对煤热解的影响
2.1 挥发分析出特性
2.1.1 CO2气化
一定温度下,CO2代替N2时,以气化方式与焦炭反应,从而增强挥发物释放,并在颗粒周围产生其他可燃气体[27]。高松平等[28]使用快速升温固定床反应器(Fixed Bed Reactor,FBR)对纯CO2气氛下褐煤热解特性进行研究,结果表明CO2的引入促进了羟基、甲基、亚甲基等脱落和芳香结构裂解,使气体产率增加。RATHNAM等[29]使用DTF研究了N2和CO2对煤炭热解的影响。结果表明,与N2相比,CO2气氛中挥发分产率高出4%~24%。CO2中较高的挥发分产率归因于气化,这与ZELLAGUI等[30]使用DTF的研究结果一致。但CHI等[31]使用自制聚光光热反应器(Concentrating Photothermal Reactor,CPR)研究高升温速率下CO2对煤热解的影响时发现,50%以内的CO2对煤的热解有促进作用,而高浓度CO2(70%)却表现出抑制作用。环境气氛中CO2浓度过高时,其在焦炭表面会发生交联反应,进而抑制气化反应发生。
2.1.2 CO2和H2O气化
PAN等[32]使用循环流化床(Fluidized Bed Combustor,FBC)研究了O2/CO2/H2O和O2/N2/H2O下富氧燃烧特性。研究指出H2O的加入增强了焦炭的气化反应,并与焦炭-CO2气化反应形成竞争,导致H2浓度升高,CO浓度降低,原因可能是高活性的H2O占据了煤颗粒表面的活性位点,从而阻碍了CO2的交联反应并促进热解反应[31]。OUYANG等[33]使用FBR研究H2O/CO2混合气氛对煤焦反应性的影响,结果表明CO2减少使煤中H自由基消耗量减少,导致H2排放量升高,CO排放降低。学者[34-35]解释发生此种现象的主要原因是水煤气变换反应(CO H2O←→CO2 H2)作用减弱。
2.2 气化对热解焦炭性质的影响
2.2.1 CO2气化
已有研究表明,由于CO2气化反应的原因,煤粉在CO2气氛中热解会析出更多挥发分进而使焦炭内部生成更多孔洞[36],导致焦炭易获得较大比表面积[29,37-39]。
除焦炭物理性质外,CO2气化反应还会对焦炭化学性质产生影响。RIAZA等[40]研究表明,煤粉在CO2中热解形成的无序碳(C/H原子比大于10)含量大于在N2中热解时,而且产生了新的含氧反应基团。SENNECA等[41]使用DTF研究了CO2/N2混合气氛对煤炭热解的影响,结果表明,与N2气氛相比,CO2气氛中生成焦炭的羧基(—OH)和内酯(—COO—)含量更高。研究表明,焦炭的反应活性可随焦炭中官能团含量的增加而增加[39,42-46]。
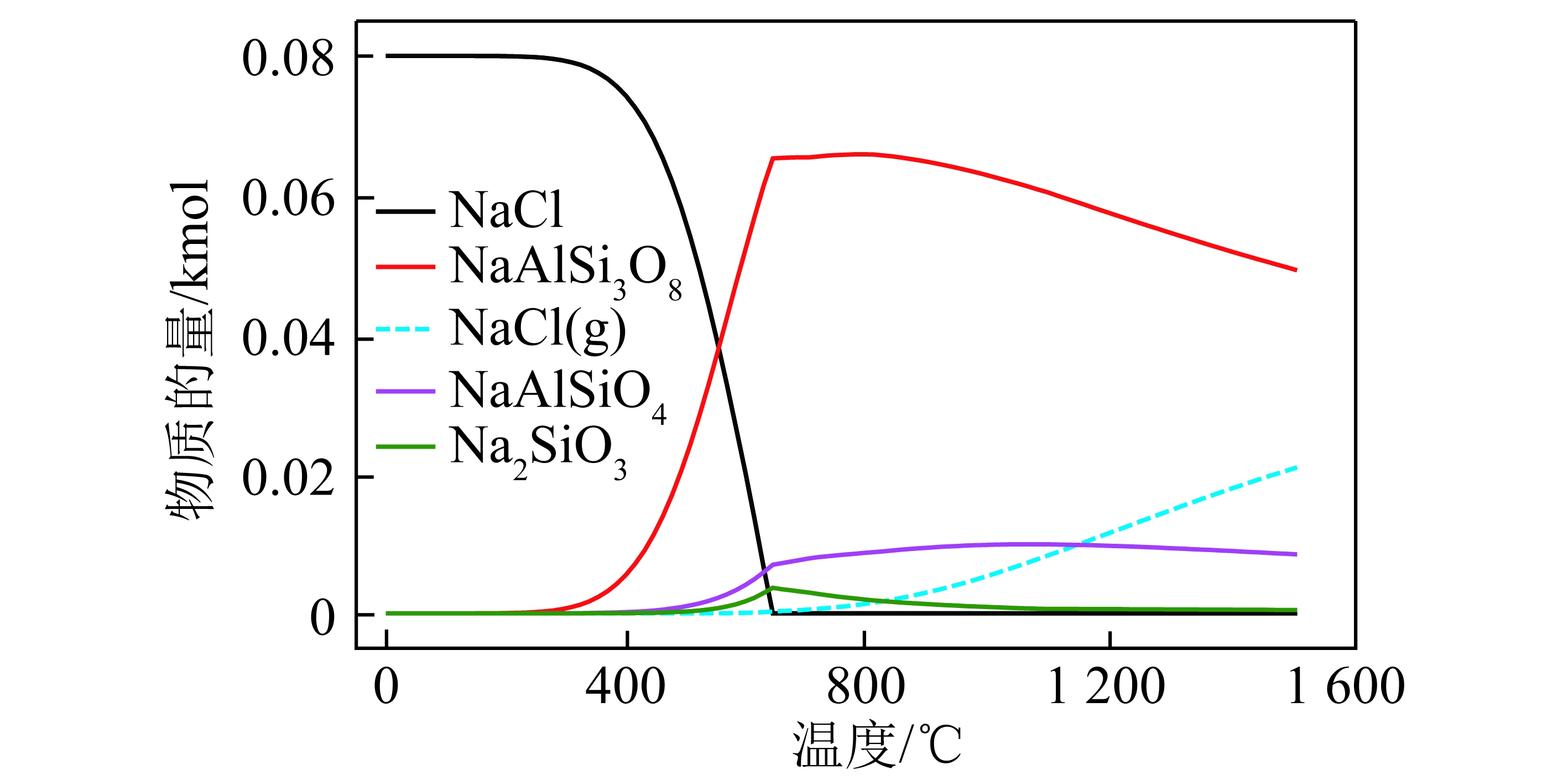
QING等[47]、高松平等[28]和XU等[48]研究了CO2和N2对焦炭产率的影响,具体如图1所示,可知随CO2比例增加,焦炭产率逐渐降低,这一现象在高温时更明显。主要原因是较高的反应温度使焦炭内外表面的活性位点和自由基等高活性物质数量增加[46],促进气化反应从而促进焦炭失重。GUIZAIN等[49]认为焦炭产率下降原因可能是CO2与焦油物质发生气化反应,进而抑制二次焦炭的形成。

图1 CO2对煤热解过程中焦炭产率的影响
Fig.1 Effect of CO2 on char yield during coal pyrolysis
2.2.2 CO2和H2O气化
CO2和H2O对焦炭的物理结构有不同影响,CO2使焦炭表面凹凸不平,而H2O则使其拥有蜂窝状空隙和较光滑的表面。在CO2气氛中生成的焦炭以微孔为主,伴有少量中孔,而在H2O/CO2和H2O气氛中,焦炭富含微孔、中孔和大孔,且孔径分布较连续[48]。OUYANG等[33]在FBR中研究发现,H2O与焦炭的气化效果显著大于CO2,不同气氛下产生的孔结构数量顺序为:H2O>CO2>N2。
焦炭产率是研究气化热解时相对表观的重要指标之一,研究表明,单独CO2或H2O气氛的气化反应会显著降低焦炭产率,但针对CO2/H2O混合气氛对焦炭产率影响的研究还较少。WANG等[50]利用ReaxFF力场模拟了H2O对煤热解的影响。结果表明在高温、高浓度H2O条件下,焦炭产率下降,高温加速了气化反应发生。ZHANG等[51]采用TGA研究了煤焦在N2/CO2/H2O中的转化率,结果表明增加H2O浓度提高了焦炭的反应性,降低了焦炭产率。QING等[47,52]和XU等[48]使用FBR研究了H2O或CO2与H2O气化对煤热解的影响,具体如图2所示,可知在纯N2和纯CO2气氛中逐步增大H2O浓度均使焦炭产率降低,但XU等[48]表明在纯N2气氛中加入H2O,对焦产率的影响明显大于纯CO2气氛加入H2O,说明H2O的气化在N2和CO2中的影响不同。推测CO2气氛中加入H2O焦炭产率下降不明显的原因可能包括CO2与H2O共同竞争活性位点,且H2O对CO2的气化产生抑制作用。

图2 CO2和H2O对煤热解过程中焦炭产率的影响
Fig.2 Effect of CO2 and H2O on char yield during coal pyrolysis
3 CO2和H2O气化对煤粉和焦炭燃烧的影响
3.1 CO2气化
O2/CO2气氛中CO2气化对燃烧过程有2个相反影响:一方面,由于CO2与焦炭气化作用可提高焦炭转化率[53-56];另一方面,气化是吸热反应,会降低煤粉颗粒燃烧时表面温度[57],从而抑制燃烧反应进行[58-60]。
LEI等[22]认为气化反应对燃烧的影响与环境气氛中氧气浓度有关。在较高氧浓度下O2/N2气氛的煤粉转化率大于O2/CO2气氛,原因可能是高氧浓度下CO2气化反应影响较小,这归因于CO2物性。在低氧浓度下O2/CO2气氛煤粉转化速率大于O2/N2气氛,这是因为在低氧浓度下CO2气化反应影响增强,气化反应对燃料碳消耗的贡献弥补了因颗粒温度降低而导致的氧化反应减弱。
气化反应是吸热反应,提高燃烧温度能促进气化反应[61]进而影响焦炭转化。HONG等[62]采用分子动力学ReaxFF-MD模拟结合试验研究了CO2气化对煤焦富氧燃烧的影响。结果表明,低氧浓度下气化反应促进煤粉燃烧。此外,KIM等[63]使用层流反应器(Laminar Entrained Flow Reactor,LEFR)和模拟研究了CO2气化对煤粉富氧燃烧可能产生的影响。结果表明,低O2浓度下CO2气化反应提高了焦炭转化率,但降低了焦炭颗粒温度,O2与CO2、焦炭反应的竞争作用会影响燃烧过程。DU等[58]通过分子模拟研究了富氧燃烧下煤炭的燃烧行为,发现O2/CO2气氛中的焦炭转化率高于O2/N2气氛。可能是由于燃烧初始阶段,O2反应活性高于CO2,所以O2更容易与焦炭表面的活性位点结合,使CO2气化反应受到明显抑制,但随焦炭孔隙率升高,比表面积增大,越来越多活性位点暴露,使O2与CO2的对活性点位的竞争作用减弱,更多的CO2可在焦炭表面活性点位吸附进而发生气化反应,可能使富氧气氛的焦炭转化率高于空气气氛。
3.2 CO2和H2O气化
CO2和H2O混合气化对煤燃烧的影响更复杂。ESCUDERO等[64-65]发现H2O浓度对煤粉着火温度有非单调影响。O2/CO2气氛中加入10% H2O后着火温度降低,但随H2O浓度增加,着火温度升高,可能是过高的H2O浓度导致焦炭比表面积减少[48]。XU等[66]使用FFBRS研究了O2/N2和O2/CO2气氛中H2O对煤粉着火特性的影响,发现加入少量水蒸气(0~5%)使燃点提前。可能的原因是环境气氖中—OH自由基随水蒸气的加入而增多,而—OH自由基对挥发性烷烃氧化起促进作用。但随H2O浓度升高,煤粉燃点延后,主要原因是H2O和CO2对焦炭的协同气化作用使焦炭表面气化反应更强烈,由于气化反应的吸热特性[67]导致煤颗粒表面温度降低,H2O大量加入使煤粉点火延迟,这一结果与其他学者[68-70]的结论类似。
LEI等[22]比较了煤粉在O2/N2、O2/CO2和O2/CO2/H2O中的燃烧特性。发现低氧浓度下由于O2/CO2气氛中气化反应的原因,煤粉转化率大于O2/N2气氛,O2/CO2气氛中一部分CO2被H2O替换后,煤粉转化率进一步增加,主要原因是CO2和H2O共气化反应进一步加速煤焦转化。
4 加压条件下气化对煤热解和焦炭燃烧的影响
4.1 热解特性
关于加压条件下气化反应对煤热解的影响,CHEN等[71]使用PTGA研究了压力和CO2气氛对煤热解的影响。随压力升高,脱挥发分速率加快,CO2引入和压力增加对煤炭脱挥发分有促进作用。ZHANG等[37]使用PDTF研究了CO2和N2气氛对煤热解的影响。研究表明气氛、压力、煤阶对煤热解有重要影响。对于烟煤,随压力增大,CO2和N2气氛中挥发分产率均降低;对于褐煤,在N2气氛中随压力升高,挥发分产率降低,但CO2气氛中挥发分产率随压力升高而上升,这是由于加压促进CO2与焦炭中大分子有机物发生气化反应,从而增强挥发分释放。
关于加压气化对焦炭性质的影响,白永辉[72]使用PFBR研究了CO2和惰性气氛(Ar)对煤焦产率的影响。结果表明,CO2气氛下热解时,由于气化作用煤焦产率随热解压力的升高而单调增加,而Ar气氛下的焦产率则随热解压力的升高而单调降低;但随热解压力增加2种气氛焦产率差异逐渐缩小,导致CO2气氛焦产率逐渐与Ar气氛下接近。LEI等[73]使用PDTF研究了加压条件下CO2对焦炭中含N和S官能团的影响,结果见表2。由表2可知,随CO2分压增加,硫化物、亚砜和砜含量降低,噻吩含量增加,这是因为高压促进焦炭-CO2气化反应,导致CO浓度升高,促进CO S![]() COS反应,降低煤焦中总硫含量。
COS反应,降低煤焦中总硫含量。
表2 CO2气氛中不同压力下气化炭中含氮官能团含量
Table 2 Content of nitrogen-containing functional groups in gasification carbon under different pressures in CO2 atmosphere

关于CO2和H2O混合气氛加压气化对热解的研究不多。ZHANG等[74]和LI等[75]分别使用PTGA和PFBR研究了加压和H2O/CO2混合气氛等对煤炭气化特性的研究,如图3所示,可知随压力增加,H2O和CO2与焦炭的气化反应速率增大,导致焦炭产率降低。

图3 压力对煤热解过程中不同气氛下焦炭产率的影响
Fig.3 Effect of pressure on char yield under different atmospheres during coal pyrolysis
4.2 燃烧特性
压力升高后气体物理性质发生明显改变,进而作用于煤粉或焦炭燃烧过程。使用PDTF研究O2/N2和O2/CO2混合气氛对煤粉燃烧的影响如图4所示,可知压力升高促进煤粉燃尽。此外,5%和10%氧浓度的O2/N2下燃尽度均高于O2/CO2,归因于CO2和N2的物性差异。

图4 1 073 K不同压力下煤粉在O2/N2和O2/CO2中的转化率
Fig.4 Conversion of pulverized coal in O2/N2 and O2/CO2 at different pressures at 1 073 K
对于加压条件下气化反应对煤燃烧特性的影响,LI等[76]使用加压流化床(Pressurized Fluidized Bed,PFB)研究发现,随压力增加,煤颗粒燃烧时颗粒峰值温度显著升高,燃尽时间缩短,气化反应可显著提高焦炭转化率,特别在高压、高氧浓度和高温条件下。HONG等[77]使用反作用力场分子动力学(ReaxFF MD)模拟研究了加压富氧燃烧过程中焦炭-CO2气化对焦炭转化率的影响,结果表明在空气(O2/N2)、富氧气氛(O2/CO2)气氛和N2/CO2混合气氛下,焦炭转化率随总压的增加而增加。HONG等[78]使用PFBR和同位素示踪法研究加压富氧燃烧对煤焦气化的影响,结果表明焦炭转化率随压力增加而提高,加压增大了O2分压从而增强了焦炭和O2的氧化反应,加压也增强了CO2与焦炭的气化反应,且O2分压升高对氧化反应的促进作用相比O2浓度升高引起的颗粒温度升高对气化反应的促进作用更显著。PDTF研究表明,在不同气氛下,煤炭转化率随压力升高而上升(图5)。在5%和10%氧浓度下,O2/N2气氛焦炭转化率大于O2/CO2气氛,说明较高的氧浓度抑制了CO2与焦炭的气化反应,由表1可知,由于O2在CO2中的扩散系数低较低,阻碍了O2与焦炭的氧化反应,从而导致煤粉在O2/CO2气氛中焦炭转化率较低。氧气体积分数为2%时,气化反应对煤焦消耗的贡献超过因颗粒温度下降而导致的氧化速率降低,所以在2% O2浓度下,O2/CO2气氛中焦炭转化率较O2/N2气氛更高。富氧气氛中H2O在加压条件下对燃烧气化影响研究很少,是未来研究方向。

图5 1 473 K、不同压力下O2/N2与O2/CO2对焦炭转化率的影响
Fig.5 Effect of O2/N2 and O2/CO2 on coke conversion under different pressures at 1 473 K
5 结语及展望
富氧燃烧中,环境气氛中较高的CO2和H2O浓度促使CO2和H2O气化反应作用于煤的整个反应过程,特别在加压富氧燃烧中,压力升高可能强化CO2和H2O气化反应的影响。研究表明,CO2单气化和CO2/H2O共气化通过与煤焦反应均可提高煤热解时挥发分中轻质气体的产率,但相应降低了焦炭产率,在加压时这种现象更明显。煤或焦炭燃烧过程中,CO2气化在温度较高和氧气浓度较低时可促进燃料碳的消耗,且H2O加入进一步加速了这一过程。随环境压力升高,CO2气化反应对燃料碳的消耗贡献逐渐增大。
目前,对于富氧燃烧中CO2和H2O气化作用下煤反应过程研究较多,但仍需重点关注以下方面:① 加压条件下,CO2/H2O共气化作用下煤热解反应动力学和机理及其对焦炭物理化学性质的影响;② 常压及加压条件下O2燃烧与CO2/H2O气化共同作用时煤焦的反应动力学和机理;③ CO2/H2O共气化作用下煤热解和焦炭燃烧的反应机理简化及其与计算流体动力学的耦合计算。
[1] 谢克昌, 赵炜. 煤化工概论[M]. 北京: 化学工业出版社, 2012.
[2] 胡鞍钢. 中国实现2030年前碳达峰目标及主要途径[J]. 北京工业大学学报(社会科学版), 2021, 21(3): 1-15.
HU Angang. China′s goal of achieving carbon peak by 2030 and its main approaches[J]. Journal of Beijing University of Technology (Social Sciences Edition), 2021, 21(3): 1-15.
[3] ABRAHAM B M,ASBURY J G,LYNCH E P,et al.Coal-oxygen process provides CO2 for enhanced recovery[J].Oil Gas Journal,1982,80(11):68-75.
[4] 孔润娟, 李伟, 任强强, 等. 加压循环流化床富氧燃烧中试研究[J]. 洁净煤技术, 2021, 27(2): 225-230.
KONG Runjuan, LI Wei, REN Qiangqiang, et al. Pilot-scale study on pressurized oxy-fuel combustion in circulating fluidized bed[J]. Clean Coal Technology, 2021, 27(2): 225-230.
[5] FASSBENDER A G. Power system with enhanced thermodynamic efficiency and pollution control:US6196000B1[P].2001-03-06.
[6] 张珈晨, 廖国远, 韩旭辉. 基于Aspen Plus的含油污泥热解过程模拟[J]. 当代化工研究, 2023(5): 31-33.
ZHANG Jiachen, LIAO Guoyuan, HAN Xuhui. Simulation of pyrolysis process of oily sludgebased on aspen plus[J]. Modern Chemical Research, 2023(5): 31-33.
[7] 李林, 段伦博, 武万强, 等. 煤颗粒流化床增压富氧燃烧脱挥发分模型[J]. 煤炭学报, 2022, 47(11): 3906-3913.
LI Lin, DUAN Lunbo, WU Wanqiang, et al. Model on devolatilization of coal particle in fluidized bed under pressurized oxy-fuel combustion[J]. Journal of China Coal Society, 2022, 47(11): 3906-3913.
[8] BORREGO A G, ALVAREZ D. Comparison of chars obtained under oxy-fuel and conventional pulverized coal combustion atmospheres[J]. Energy &Fuels, 2007, 21(6): 3171-3179.
[9] NAEDI P, PISUPATI S. Effect of CO2 during coal pyrolysis and char burnout in oxy-coal combustion[J]. Energy &Fuels, 2011, 25(6): 2452-2459.
[10] 张洪, 赵博, 陈佳宝, 等. N2/CO2气氛下内在矿物对煤焦反应活性的影响[J]. 燃烧科学与技术, 2012, 18(5): 393-397.
ZHANG Hong, ZHAO Bo, CHEN Jiabao, et al. Influence of included minerals on the intrinsic reactivity of chars prepared under N2 and CO2 atmosphere[J]. Journal of Combustion Science and Technology, 2012, 18(5): 393-397.
[11] BEJARANO P A, LEVENDIS Y A. Single-coal-particle combustion in O2/N2 and O2/CO2 environments[J]. Combustion and Flame, 2008, 153(1/2): 270-287.
[12] ESCUDERO A I, AZNAR M, D EZ L I, et al. From O2/CO2 to O2/H2O combustion: The effect of large steam addition on anthracite ignition, burnout and NOx formation[J]. Fuel Processing Technology, 2020, 206: 106432.
EZ L I, et al. From O2/CO2 to O2/H2O combustion: The effect of large steam addition on anthracite ignition, burnout and NOx formation[J]. Fuel Processing Technology, 2020, 206: 106432.
[13] SHADDIX C R, MOLINA A. Particle imaging of ignition and de-volatilization of pulverized coal during oxy-fuel combustion[J]. Proceedings of the Combustion Institute, 2009, 32(2): 2091-2098.
[14] BETANCUR Y,S NCHEZ A, BUENO-L
NCHEZ A, BUENO-L PEZ A, et al. Impact of biomass and main biomass components on coal reactivity under oxy-combustion conditions:A comparison of physicoche-mical char properties obtained under N2 and CO2 atmospheres[J]. Energy &Fuels, 2017, 31(5): 5603-5611.
PEZ A, et al. Impact of biomass and main biomass components on coal reactivity under oxy-combustion conditions:A comparison of physicoche-mical char properties obtained under N2 and CO2 atmospheres[J]. Energy &Fuels, 2017, 31(5): 5603-5611.
[15] GIL M V, RIAZA J,  LVAREZ L, et al. A study of oxy-coal combustion with steam addition and biomass blending by thermogravimetric analysis[J]. Journal of Thermal Analysis and Calorimetry,2012, 109(1): 49-55.
LVAREZ L, et al. A study of oxy-coal combustion with steam addition and biomass blending by thermogravimetric analysis[J]. Journal of Thermal Analysis and Calorimetry,2012, 109(1): 49-55.
[16] BUHRE B J P, ELLIOTT L K, SHENG C D,et al. Oxy-fuel combustion technology for coal-fired power generation[J]. Progress in Energy and Combustion Science, 2005, 31(4): 283-307.
[17] 黄晓宏, 皮理刚, 柳朝晖, 等. 富氧燃烧条件下煤焦的燃尽特性[J]. 工程热物理学报, 2015, 36(2): 441-444.
HUANG Xiaohong, PI Ligang, LIU Zhaohui, etal. Burnout characteristic of coal chars under oxyfuel combustion conditions[J]. Journal of Engineering Thermophysics, 2015, 36(2): 441-444.
[18] RIAZ J,  LVAREZ L, GIL M V, et al. Effect of oxy-fuel combustion with steam addition on coal ignition and burnout in an entrained flow reactor[J]. Energy, 2011, 36(8): 5314-5319.
LVAREZ L, GIL M V, et al. Effect of oxy-fuel combustion with steam addition on coal ignition and burnout in an entrained flow reactor[J]. Energy, 2011, 36(8): 5314-5319.
[19] CHEN L, YONG S Z, GHONIEM A F. Oxy-fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling[J]. Progress in Energy and Combustion Science, 2012, 38(2): 156-214.
[20] GONZALO-TIRADO C, JIMÉNEZ S, BALLESTER J. Gasification of a pulverized sub-bituminous coal in CO2 at atmospheric pressure in an entrained flow reactor[J]. Combustion and Flame, 2012, 159(1): 385-395.
[21] ZHANG L, WU D, CAI L, et al. The chemical and physical ef-fects of CO2 on the homogeneous and heterogeneous ignition of the coal particle in O2/CO2 atmospheres[J]. Proceedings of the Combustion Institute, 2017, 36(2): 2113-2121.
[22] LEI M, ZOU C, XU X B, et al. Effect of CO2 and H2O on the combustion characteristics and ash formation of pulverized coal in oxy-fuel conditions[J]. Applied Thermal Engineering, 2018, 133: 308-315.
[23] CAI L, ZOU C, GUAN Y W, et al. Effect of steam on ignition of pulverized coal particles in oxy-fuel combustion in a drop tube furnace[J]. Fuel, 2016, 182: 958-966.
[24] KOPS R B, PEREIRA F M, RABAÇAL M, et al. Effect of ste-am on the single particle ignition of solid fuels in a drop tube furnace under air and simulated oxy-fuel conditions[J]. Proceedings of the Combustion Institute, 2019, 37(3): 2977-2985.
[25] YI B J, ZHANG L Q, HUANG F, et al. Effect of H2O on the combustion characteristics of pulverized coal in O2/CO2 atmosphere[J]. Applied Energy, 2014, 132: 349-357.
[26] LI Y K, FENG D D, SUN S Z, et al. Mechanism of CO2 and H2O action on char in oxy-fuel combustion with wet flue gas recirculation[J]. Energy &Fuels, 2023, 37(14): 10721-10725.
[27] ZHANG L, BINNER E, QIAO Y, et al. In situ diagnostics of Victorian brown coal combustion in O2/N2 and O2/CO2 mixtures in drop-tube furnace[J]. Fuel, 2010, 89(10): 2703-2712.
[28] 高松平, 赵建涛, 王志青, 等. CO2对褐煤热解行为的影响[J]. 燃料化学学报, 2013, 41(3): 257-264.
GAO Songping, ZHAO Jiantao, WANG Zhiqing, et al. Effect of CO2 on pyrolysis behaviors of lignite[J]. Journal of Fuel Chemistry and Technology, 2013, 41(3): 257-264.
[29] RATHNAM R K, ELLIOTT L K, WALL T F, et al. Differences in reactivity of pulverised coal in air (O2/N2) and oxy-fuel (O2/CO2) conditions[J]. Fuel Processing Technology, 2009, 90(6): 797-802.
[30] ZELLAGUI S, SCHÖNNENBECK C, ZOUAOUI N, et al. Fast pyrolysis of coals under N2 and CO2 atmospheres[J]. Journal of Thermal Analysis and Calorimetry, 2018, 133(3): 1535-1547.
[31] CHI H Y, LI H J, SU S, et al. Effects of CO2 and H2O on coal pyrolysis with the ultrafast heating rate in a concentrating photothermal reactor[J]. Journal of the Energy Institute, 2021, 98: 44-52.
[32] PAN F, ZHU J G, LIU J Z, et al. Effect of H2O on preheating combustion characteristics in O2/CO2 and O2/N2 atmospheres[J]. Journal of Thermal Science, 2023, 32(6): 2235-2242.
[33] OUYANG J C, HONG D K, JIANG L K, et al. Effect of CO2 and H2O on char properties. Part 1: Pyrolysis char structure and reactivity[J]. Energy &Fuels, 2020, 34(4): 4243-4250.
[34] PRABOWO B, UMEKI K, YAN M, et al. CO2-steam mixture for direct and indirect gasification of rice straw in a downdraft gasifier: Laboratory-scale experiments and performance prediction[J]. Applied Energy, 2014, 113: 670-679.
[35] UMEKI K, NAMIOKA T, YOSHIKAWA K. The effect of steam on pyrolysis and char reactions behavior during rice straw gasification[J]. Fuel Processing Technology, 2012, 94(1): 53-60.
[36] DU Y B, ZHAO N, WANG J X, et al. Effect of CO2 on coal devolatilization in oxy-fuel combustion: Exploring the influence of temperature and inherent alkali/alkaline earth metal[J]. International Journal of Greenhouse Gas Control, 2020, 96: 103004.
[37] ZHANG J Y, CHEN C M, ZHOU A, et al. Morphology of char particles from coal pyrolysis in a pressurized entrained flow reactor: Effects of pressure and atmosphere[J]. Energy, 2022, 238: 121846.
[38] BANERJEE N N, MURTY G S, RAO H S, et al. Flash pyrolysis of coal: Effect of nitrogen, argon and other atmospheres in increasing olefin concentration and its significance on the mechanism of coal pyrolysis[J]. Fuel, 1973, 52(3): 168-170.
[39] BAI Y H, WANG Y L, ZHU S H, et al. Structural features and gasification reactivity of coal chars formed in Ar and CO2 atmospheres at elevated pressures[J]. Energy, 2014, 74: 464-470.
[40] RIAZA J,  LVAREZ L, GIL M V, et al. Effect of oxy-fuel combustion with steam addition on coal ignition and burnout in an entrained flow reactor[J]. Energy, 2011, 36(8): 5314-5319.
LVAREZ L, GIL M V, et al. Effect of oxy-fuel combustion with steam addition on coal ignition and burnout in an entrained flow reactor[J]. Energy, 2011, 36(8): 5314-5319.
[41] SENNECA O, VOROBIEV N, WÜTSCHER A, et al. Assessm-ent of combustion rates of coal chars for oxy-combustion applications[J]. Fuel, 2019, 238: 173-185.
[42] SHENG C D. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity[J]. Fuel, 2007, 86(15): 2316-2324.
[43] TAY H L, LI C Z. Changes in char reactivity and structure during the gasification of a Victorian brown coal: Comparison between gasification in O2 and CO2[J]. Fuel Processing Technology, 2010, 91(8): 800-804.
[44] ZHANG L, KAJITANI S, UMEMOTO S, et al. Changes in nascent char structure during the gasification of low-rank coals in CO2[J]. Fuel, 2015, 158: 711-718.
[45] LI Z H, ZOU R J, HONG D K, et al. Effect of CO2 and H2O on char properties. part 2: In situ and Ex situ char in oxy-steam combustion[J]. Energy &Fuels, 2020, 34(6): 7554-7563.
[46] RINC N PRAT S, SCHNEIDER C, KOLB T. Determination of active sites during gasification of biomass char with CO2 using temperature-programmed desorption. Part 2: Influence of ash components[J]. Fuel, 2020, 267: 117179.
N PRAT S, SCHNEIDER C, KOLB T. Determination of active sites during gasification of biomass char with CO2 using temperature-programmed desorption. Part 2: Influence of ash components[J]. Fuel, 2020, 267: 117179.
[47] QING M X, SU S, GAO J, et al. Effects of CO2/H2O on the characteristics of chars prepared in CO2/H2O/N2 atmospheres[J]. Fuel Processing Technology, 2018, 173: 262-269.
[48] XU J, SU S, SUN Z J, et al. Effects of H2O gasification reaction on the characteristics of chars under oxy-fuel combustion conditions with wet recycle[J]. Energy &Fuels, 2016, 30(11): 9071-9079.
[49] GUIZANI C, ESCUDERO Sanz F J, SALVADOR S. Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties[J]. Fuel, 2014, 116: 310-320.
[50] WANG M, GAO J M, XU J J, et al. Effect of H2O on the transformation of sulfur during demineralized coal pyrolysis: Molecular dynamics simulation using ReaxFF[J]. Energy &Fuels, 2021, 35(3): 2379-2390.
[51] ZHANG Z H, YI B J, SUN Z S, et al. Reaction process and cha-racteristics for coal char gasification under changed CO2/H2O atmosphere in various reaction stages[J]. Energy, 2021, 229: 120677.
[52] QING M X, SU S, CHI H Y, et al. Relationships between structural features and reactivities of coal-chars prepared in CO2 and H2O atmospheres[J]. Fuel, 2019, 258: 116087.
[53] LEI M, SUN C, WANG C B. Effect of CO2 and H2O gasi-fications on the burning behavior and NO release process of pulverized coal at low oxygen concentrations during oxy-fuel combustion[J]. Journal of Energy Engineering, 2019, 145(2):04019003.
[54] ZHANG L, BINNER E, QIAO Y, et al. In situ diagnostics of Victorian brown coal combustion in O2/N2 and O2/CO2 mixtures in drop-tube furnace[J]. Fuel, 2010, 89(10): 2703-2712.
[55] 雷鸣, 黄星智, 王春波. CO2/H2O气化反应作用下煤恒温富氧燃烧特性[J]. 煤炭学报, 2016, 41(S2): 536-541.
LEI Ming, HUANG Xingzhi, WANG Chunbo. Oxy-fuel combustion characteristics of pulverized coal under the cction of CO2/H2O gasification at constant temperature[J]. Journal of China Coal Society, 2016, 41(S2): 536-541.
[56] 丁继伟.富氧气氛下水蒸气对煤焦燃烧的影响[M].武汉:华中科技大学,2016.
[57] GEIER M, SHADDIX C R, DAVIS K A, et al. On the use of single-film models to describe the oxy-fuel combustion of pulverized coal char[J]. Applied Energy, 2012, 93: 675-679.
[58] DU Y B, WANG C A, XIN H H, et al. Atomistic simulation of coal char oxy-fuel combustion: quantifying the influences of CO2 to char reactivity[J]. Energy &Fuels, 2019, 33(10): 10228-10236.
[59] WANG W K, BU C S, G MEZ-BAREA A, et al. O2/CO2 and O2/N2 combustion of bituminous char particles in a bubbling fluidized bed under simulated combustor conditions[J]. Chemical Engineering Journal, 2018, 336: 74-81.
MEZ-BAREA A, et al. O2/CO2 and O2/N2 combustion of bituminous char particles in a bubbling fluidized bed under simulated combustor conditions[J]. Chemical Engineering Journal, 2018, 336: 74-81.
[60] LI L L, ZHANG T H, CAI J H, et al. Operando spectroscopic and isotopic-label-directed observation of LaN-promoted Ru/ZrH2 catalyst for ammonia synthesis via associative and chemical looping route[J]. Journal of Catalysis, 2020, 389: 218-228.
[61] TOFTEGAARD M B, BRIX J, JENSEN P A, et al. Oxy-fuel combustion of solid fuels[J]. Progress in Energy and Combustion Science, 2010, 36(5): 581-625.
[62] HONG D K, SI T, LI X X, et al. Reactive molecular dynamic simulations of the CO2 gasification effect on the oxy-fuel combustion of Zhundong coal char[J]. Fuel Processing Technology, 2020, 199: 106305.
[63] KIM D, CHOI S, SHADDIX C R, et al. Effect of CO2 gasificati-on reaction on char particle combustion in oxy-fuel conditions[J]. Fuel, 2014, 120: 130-140.
[64] ESCUDERO A I, AZNAR M, D EZ L I. Oxy-steam combustion: The effect of coal rank and steam concentration on combustion characteristics[J]. Fuel, 2021, 285: 119218.
EZ L I. Oxy-steam combustion: The effect of coal rank and steam concentration on combustion characteristics[J]. Fuel, 2021, 285: 119218.
[65] ESCUDERO A I, AZNAR M, D EZ L I, et al. From O2/CO2 to O2/H2O combustion: The effect of large steam addition on anthracite ignition, burnout and NOx formation[J]. Fuel Processing Technology, 2020, 206: 106432.
EZ L I, et al. From O2/CO2 to O2/H2O combustion: The effect of large steam addition on anthracite ignition, burnout and NOx formation[J]. Fuel Processing Technology, 2020, 206: 106432.
[66] XU Y, LI S Q, YAO Q, et al. Investigation of steam effect on ignition of dispersed coal particles in O2/N2 and O2/CO2 ambiences[J]. Fuel, 2018, 233: 388-395.
[67] MAREK E,  B. Experimental studies of single particle combustion in air and different oxy-fuel atmospheres[J]. Applied Thermal Engineering, 2014, 66(1/2): 35-42.
B. Experimental studies of single particle combustion in air and different oxy-fuel atmospheres[J]. Applied Thermal Engineering, 2014, 66(1/2): 35-42.
[68] LIU X Q, LIU Y H, WANG B, et al. Experimental study on ignition delay and reaction characteristics of Zhundong coal particles in O2/CO2/H2O atmosphere[J]. Energy &Fuels, 2020, 34(6): 7465-7476.
[69] MA L, CHEN X K, YU S H, et al. Effect of H2O on the combustion characteristics and interactions of blended coals in O2/H2O/CO2 atmosphere[J]. Journal of the Energy Institute, 2021, 94: 222-232.
[70] DUESO C, MAYORAL M C, ANDRÉS J M, et al. Towards oxy-steam combustion: The effect of increasing the steam concentration on coal reactivity[J]. Fuel, 2019, 239: 534-546.
[71] CHEN H P, YANG H P, JU F D, et al. The influence of pressure and temperature on coal pyrolysis/gasification[J]. Asia-Pacific Journal of Chemical Engineering, 2007, 2(3): 203-212.
[72] 白永辉.CO2作为气化剂对煤焦-H2O气化反应的影响机制[M].太原:太原理工大学,2014:55-56.
[73] LEI M, ZHANG Y C, HONG D K, et al. Characterization of nitrogen and sulfur migration during pressurized coal pyrolysis and oxy-fuel combustion[J]. Fuel, 2022, 317: 123484.
[74] ZHANG R, WANG Q H, LUO Z Y, et al. Coal char gasification in the mixture of H2O, CO2, H2, and CO under pressured conditions[J]. Energy &Fuels, 2014, 28: 832-839.
[75] LI F H, YAN Q X, HUANG J J, et al. Lignite-char gasification mechanism in mixed atmospheres of steam and CO2 at different pressures[J]. Fuel Processing Technology, 2015, 138: 555-563.
[76] LI L, DUAN L B, YANG Z H, et al. Pressurized oxy-fuel combustion of a char particle in the fluidized bed combustor[J]. Proceedings of the Combustion Institute, 2021, 38(4): 5485-5492.
[77] HONG D K, WANG Y D, ZHAI X M. A ReaxFF study of the effect of pressure on the contribution of char-CO2 gasification to char conversion during pressurized oxy-fuel combustion[J]. Fuel, 2022, 329: 125439.
[78] HONG P, LI L, DUAN Y Q, et al. Gasification decoupling during pressurized oxy-coal combustion by the isotope tracer method[J]. Energy &Fuels, 2022, 36(6): 3239-3246.
Research progress on the effects of CO2 and H2O gasification reactions on coal pyrolysis and char combustion in oxygen-rich atmosphere

移动阅读