“第四届循环流化床锅炉国际会议”专题
fast-reaction thermogravimetric analysis for kinetic studies of Ca(OH)2/CaO fluidized thermochemical heat storage
0 Introduction
The rapid development of renewable energy has led to higher requirements for large-scale energy storage.Thermal energy storage is an important method that includes sensible[1], latent[2], and thermochem-ical heat storage[3]. Thermochemical heat storage uses endothermic and exothermic processes of reversible chemical reactions to store and release energy. Previous studies have shown that the Ca(OH)2/CaO material system has potential for large-scale industrial applications owing to its low cost, high energy storage density, non-toxicity, reversibility, and fast reaction kinetics[4]. In addition, the use of industrial solid waste carbide slag with Ca(OH)2 as the main thermochemical heat storage material has been proposed[5-6].
Fixed-bed reactors are suitable for Ca(OH)2/CaO thermochemical heat storage[7-13]. Thermogravimetric analysis (TGA) has been used to explore the reaction kinetic characteristics[14-19]. With the development of large-scale energy storage technology, fluidized bed reactors for Ca(OH)2/CaO thermochem-ical heat storage have become popular choice[20-25].
Since the heat transfer rate of the particles during fluidization is high, the heat transfer area required by the fluidized bed reactors is markedly lower than that of the fixed-bed reactors. When the particles are in a fluidized state, the mass transfer resistance caused by the stacked particles can be significantly reduced, further increasing the reaction rate. Because the reaction kinetics of Ca(OH)2/CaO under fluidization directly affect the heat storage rate of the energy storage system and the design of the fluidized bed reactor, the reaction kinetics of Ca(OH)2/CaO under fluidization have attracted much attention for research.
Existing kinetic studies on Ca(OH)2/CaO thermochemical heat storage have been performed using TGA[14-19]. TGA adopts the temperature-programmed method. Its heating rate is notably lower than that of the particles under fluidization, which means that it cannot provide constant-temperature reaction conditions for Ca(OH)2. YIN et al.[26] demonstrated that the difference in the heating conditions provided by TGA and the micro-fluidized bed reaction analyzer for the sample would cause different reaction kinetic characteristics. JIANG et al.[27] demonstrated that the mass transfer inhibition phenomenon in TGA was significant, rendering TGA unable to provide samples with excellent mass transfer conditions, such as a fluidized bed. Therefore, the reaction kinetic data obtained by TGA have a limited reference value for Ca(OH)2/CaO fluidized thermochemical heat storage research. CRIADO et al.[28] designed a TG system to study the intrinsic characteristics of the hydration and dehydration reactions of CaO particles. Because Ca(OH)2 decomposes during the heating stage, the hydration reaction of CaO must firstly be studied, followed by the decomposition of Ca(OH)2. However, that equipment cannot directly study the fast reaction of Ca(OH)2 or carbide slag. The micro-fluidized bed reaction analyzer can provide fluidized conditions for the sample and relies on the gas signal (detected by a Mass Spectrometer or Fourier-Transform Infrared (FTIR) Spectrometer) at the reactor outlet for reaction kinetic studies[29]. Due to the back-mixing and axial diffusion of components in the fluidized bed reactor, the gas flow will deviate from the plug flow, and the gas concentration signal obtained by the gas analyzer will be distorted to a certain extent. For fast gas-solid reactions (such as the decomposition of Ca(OH)2), the distortion of gas signal will be more severe. Moreover, the gas signals of the heat storage and release processes of Ca(OH)2/CaO are assigned to water vapor, which is difficult to be detected accurately by a Mass Spectrometer or FTIR Spectrometer owing to the difficulty in obtaining standard gases and other factors. Therefore, using a micro-fluidized bed reaction analyzer for Ca(OH)2/CaO fluidized thermochemical heat storage research is difficult.
At present, no equipment can study the reaction kinetics of Ca(OH)2/CaO under fluidization, which limits development in this area. A fast-reaction TGA technique has been designed by author′s research team to solve this problem. This novel technique provides Ca(OH)2/CaO with heat and mass transfer conditions closer to those of fluidized bed reactors. Compared with TGA, fast-reaction TGA provides better heat and mass transfer conditions for the sample by rapidly moving the high-temperature reactor and implementing high-speed purge gas to quickly reach the set temperature and reduce the mass transfer resistance during the reaction, allowing for reliable mass signals to be obtained for conducting kinetic studies. Therefore, fast-reaction TGA can be used to directly study the fast reaction of Ca(OH)2 or carbide slag.
The composition of the fast-reaction TGA equipment was introduced, and the influence of operating conditions on the performance of the fast-reaction TGA was researched to determine several key operating parameters. An in-depth performance verification will be conducted in future.
1 Material and methods
1.1 Fast-reaction TGA
Fast-reaction TGA measures the sample mass over time in a certain temperature environment. The composition, thermal stability, thermal decomposition, and other information related to the sample mass can be obtained by analyzing the TG curve. Compared with TGA, the biggest advantage of the fast-reaction TGA is that it can measure the changes in sample mass with time at a high heating rate after the sample enters a high-temperature environment.
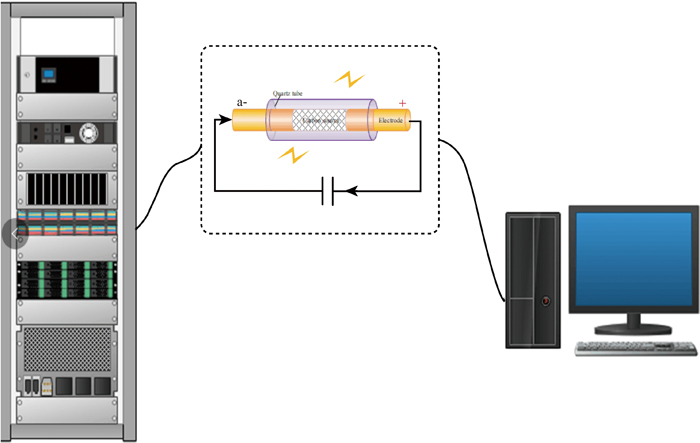
The equipment for fast-reaction TGA consisted of a reactor, weighing module, compressed gas system, steam generation system, and online temperature and mass data acquisition system (Fig. 1). The reactor (1 200 lift tube furnace, Shanghai YuZhi Science and Technology Co., Ltd., China) could move at a set speed on the sliding rail. The temperature of the reactor was controlled using an in-reactor thermocouple (GG-K-30-SLE-BULK, OMEGA, USA). When the reactor descended to the designated location, this thermocouple was placed above the quartz crucible to ensure that the temperature at the position where the quartz crucible located met the temperature requirements. The upper end of the tube (310S stainless-steel) in the reactor was closed, and the lower end was open. The weighing module (WKX204, METTLER TOLEDO, Swiss) was controlled by a computer for the online collection of mass data. When there were no special instructions, the sampling frequency was set to 45 per second for performance testing. A cooling jacket was set between the weighing module and reactor, and a chiller (HS-DC600, Beijing Hongshengboyuan Technology Co., Ltd., China) provided cooling circulating water to ensure that the weighing module was not affected by the high-temperature reactor. The weighing module was equipped with a clamping head to hold a quartz rod. The top of the quartz rod was a quartz tray meant for holding a quartz crucible. The thermocouple temperature measurement point was placed under the quartz crucible, not in contact with the quartz crucible. This feature was a novel design different from that of TGA, and hence, could provide a more precise temperature near the samples. The thermocouple was passed through the hollow quartz rod to connect to the temperature transfer module (DAM-3038, Beijing Art Technology Development Co., Ltd., China). A computer recorded the temperature data in real-time. The compressed gas system consisted of a compressed gas cylinder, mass flowmeter (ACU10FD-LC, Beijing AccuFlow Technology Co., Ltd., China), and heater. After preheating, the gas entered the stainless-steel coil in the reactor to continue to absorb heat, ensuring that the temperature at which the gas reached the surface of the quartz crucible was consistent with the set temperature in the reactor. A steam generation system was developed for further research on the exothermic process of CaO hydration. The injection pump (TYD01-01, Lead Fluid Technology Co., Ltd., China) injected liquid water into the vaporizer (VDRT-200, Quzhou Vodo Instrument Co., Ltd., China), which generated continuous water vapor. In the heat release experiment, the water vapor was mixed with the preheated air in the mixing chamber to form moist air with a certain water vapor partial pressure, which then entered the stainless-steel coil in the reactor. The main technical parameters of the fast-reaction TGA technique are listed in Table 1.

Fig.1 Diagram of the fast-reaction TGA experimental system
Table 1 Main technical parameters of the fast-reaction TGA

The operation procedure for the fast-reaction TGA was briefly described as follows: the reactor was initially positioned at the top of the sliding rail. The reactor was heated using electricity to set the reactor temperature for the experiment. The purge gas was introduced, and the temperature in the reactor was determined to be stable by the in-reactor thermocouple. Then, a predetermined amount of the sample was placed in the quartz crucible, which was placed in the quartz tray. Mass and temperature data were collected, and an electronic control device was used to lower the reactor to the designated location at a set speed. When the mass signal no longer changed, the reaction was considered complete. The operation procedure was detailed in subsection 2.5.
1.2 Properties of Ca(OH)2 sample
Analytically pure Ca(OH)2 was used as a heat storage material to test the fast-reaction TGA technique. The specific parameters of the Ca(OH)2 sample are listed in Table 2.
Table 2 Characteristic parameters of the Ca(OH)2 sample

2 Results and discussion
2.1 Influence of airflow on heat transfer in the stainless-steel coil
To ensure that the temperature of the air flowing through the sample surface is consistent with the target temperature of the experiment, it is necessary to study the heat transfer effect of the preheated air entering the stainless-steel coil. The heater was set to 180 ℃. The K-type thermocouple in the reactor, located 5 mm directly above the quartz crucible in the vertical direction and on the same horizontal line as the center of the coil outlet section in the horizontal direction, was used to measure the temperature of the air as it passed over the surface of the Ca(OH)2 sample.
The reactor was set at different temperatures, and at each temperature, different volumes of compressed air were introduced (Table 3). The stable readings of the K-type thermocouples were then recorded. The results are presented in Fig.2.
Table 3 Air velocities of different air volumes at the different reactor temperatures


Fig.2 Temperature measurement results of the K-type thermocouple under different reactor temperatures and air volumes
The temperature measured by the K-type thermocouple increases with an increase in air volume (Fig.2), and the heat transfer effect of the air in the coil exceeds the expected value. With a constant coil size (length and diameter),air velocity will increase with an increase in air volume. On one hand, an increase in air velocity will shorten the residence time of the air in the coil, which is not conducive to sufficient heat exchange. On the other hand, an increase in air velocity will strengthen the disturbance in the coil and increase the convective heat transfer coefficient to enhance the heat transfer effect. Fig. 2 shows that heat transfer enhancement is the predominant effect observed in this experiment. Moreover, the increase in air temperature at the same air volume is similar at different reactor temperatures, indicating sufficient heat exchange.
2.2 Creation of a high heating rate in the fast-reaction TGA technique
Fast-reaction TGA facilitates a higher heating rate for the sample by the rapid movement of the high-temperature reactor, allowing the sample to reach the target temperature quickly. The temperature of the sample entering the reactor was determined as follows: firstly, the reactor temperature was adjusted to 500 ℃, which was measured using an in-reactor thermocouple. Then, the reactor was allowed to descend to the designated location at speeds of 10, 20, 30, 40, and 50 mm/s without compressed air. At the designated location, when compressed air was used in the experiment, the compressed air at the outlet of the coil flowed over the quartz crucible surface. The K-type thermocouple placed under the quartz crucible was used to measure the sample temperature. As shown in Fig. 3, when the descending speed of the reactor is ≥30 mm/s, the heating rate of the sample do not increase.

Fig.3 Sample heating curves generated at different reactor descending speeds
Fig. 4 compares the sample heating curve when the reactor adoptes a 50 mm/s descending speed at four different reactor temperatures. Data from the ambient temperature to 95% of the set temperature were used to calculate the average heating rate. The maximum instantaneous heating rate was obtained by calculating the change in the sample temperature in 1 s. As shown in Fig. 4, the higher the reactor temperature, the greater are the rates of average heating and maximum instantaneous heating. When the reactor temperatures are 450, 500, 550, and 600 ℃, the average heating rates are 1.47, 1.73, 2.30, and 2.89 ℃/s, respectively, and the maximum instantaneous heating rates are 25.14, 30.47, 33.29, and 37.49 ℃/s, respectively (Table 4). Compared with the low heating rate of the TGA technique (usually < 50 ℃/min), the heating method adopted for fast-reaction TGA can realize an approximate thermostatic reaction of Ca(OH)2.
Table 4 Comparison of temperature rise at different reactor temperatures at a reactor descending speed of 50 mm/s


Fig.4 Comparison of sample heating curves
2.3 Stability of the weighing module when operating at ambient temperature
The accuracy and stability of the weighing module are important for the fast-reaction TGA. Hence, it is necessary to study the factors that affects the reading of the weighing module during the experiment, especially the influence of the reactor′s movement on the weighing module′s readings. Firstly, peeled weighing was performed on the weighing module. The reactor was then allowed to descend to the designated location at different speeds (10, 20, 30, 40, and 50 mm/s) without heating the reactor or introducing compressed air. The weighing module was used to record the mass data. The results shown in Fig. 5 reveal that the faster the reactor descends, the smaller is the fluctuation of the weighing module′s reading. When the reactor′s descending speed is 40 or 50 mm/s, the reading fluctuation is within ±0.5 mg. After the descent of the reactor is complete, the reading quickly recovers and remains stable. Therefore, choosing a higher reactor descending speed can quickly minimize the reading fluctuation caused by the impact of air in the reactor cavity.

Fig.5 Effect of reactor descent behavior on weighing module readings at ambient temperature
The influence of the ambient-temperature airflow at the outlet of the stainless-steel coil on the reading of the weighing module was investigated. Firstly, the reactorset at ambient temperature was allowed to descend to the designated location. Subsequently, compressed air at an ambient temperature of 500 mL/min was introduced, and the weighing module recorded the mass data. As shown by the blue line in Fig. 6, the reading of the weighing module is not affected by airflow at ambient temperature. After clarifying the influences of the descent of the ambient temperature reactor and the ambient temperature airflow, these two factors were coupled to determine the comprehensive influence. The airflow was 500 mL/min and the reactor descended to the designated location at different speeds (10, 20, 30, 40, and 50 mm/s). Data from the weighing module were recorded. The results presented in Fig. 6 reveal that the faster the reactor descends, the smaller is the fluctuation in the weighing module′s reading. When the descending speed of the reactor exceeds 10 mm/s, the reading of the weighing module fluctuates within ±0.5 mg, and the reading quickly returns to stability after the reactor descends.

Fig.6 Effect of airflow (500 mL/min) and reactor speeds on weighing module reading
Therefore, regardless of the heating rate described in subsection 2.2 or the influence of the reactor movement and airflow in subsection 2.3, the descending speed of the reactor in the fast-reaction TGA technique should be 50 mm/s.
2.4 Stability of the weighing module when operating at high temperature
The results in subsection 2.3 show that the desc-ent of the reactor and airflow at ambient temperature has little impact on the weighing module. Therefore, the thermal state of the experiment situation is further considered. Under hot conditions, the air in the reactor will expand, and the density will decrease. Therefore, the descent of a high-temperature reactor will affect the reading of the weighing module. Further, the velocity of compressed air (500 mL/min) at the outlet of the coil at ambient temperature (25 ℃) is 0.17 m/s, but the air velocity increases when the temperature increases. As shown in Table 3, the velocity of 500 mL/min of compressed air at 500 ℃ is 0.43 m/s. Therefore, it is unknown that whether high-speed hot air blowing over the surface of the quartz crucible affects the reading of the weighing module. It is also necessary to confirm whether high-speed airflow blows off the sample. In summary, under hot conditions, the high-temperature air in the reactor and high-speed air at the exit of the coil may affect the reading of the weighing module. Decoupling experiments were performed to study the influence of these two factors.
To explore the influence of the high-temperature reactor, peeled weighing was firstly performed in the weighing module. Then, the reactor set at 500 ℃ was allowed to descend to the designated location at different speeds (10, 20, 30, 40, and 50 mm/s) with no compressed air introduced. Data from the weighing module were obtained. The experimental results shows that the mass change trends obtained at different reactor descending speeds are similar, and there are characteristic data points with definite meanings. Because a slower reactor descending speed will separate the characteristic data points more clearly, it is easier to confirm the meaning of the characteristic points. Therefore, only the results of the descending speed of the reactor at 10 mm/s are described here, as shown in Fig.7. The changing trend of the experimental data and the meaning of the characteristic points of other reactor descending speeds are the same, and the only difference is the degree of separation of the characteristic data points.

Fig.7 Readings of the weighing module when the reactor at 500 ℃ descends at a speed of 10 mm/s
As shown in Fig.7, before the empty crucible enters the reactor, due to the convection of the hot air in the reactor and the outside cold air, the reading of the weighing module fluctuates significantly and does not remain stable at 0. When the empty crucible enters the reactor, the reading of the weighing module begins to change regularly because the empty crucible leaves the convection zone. As the empty crucible ascends relative to the reactor, the resistance of the hot air in the reactor causes the reading of the weighing module to increase rapidly. After the empty crucible reaches the designated location, the reading of the weighing module reaches its peak. The faster the reactor descends, the shorter is the duration of the process. After the empty crucible reaches the designated location, the mass signal begins to decline, indicating that the hot air resistance effect caused by the relative movement gradually disappeares. The reading of the weighing module stabilizes at a value higher than 0, which is caused by the effect of the air temperature in the reactor, and the final values produced at different reactor temperatures are also different. Therefore, the effect of the blank experimental values should be considered when processing the experimental results. When the reactor ascends, the reading of the weighing module decreases rapidly. This is because the empty crucible descends relative to the reactor, and the air in the reactor gives the empty crucible an upward buoyancy force, causing the reading of the weighing module to decrease. When the empty crucible leaves the reactor, it is affected by the convection of hot and cold air, and the mass data begins to fluctuate.
It was studied that whether the high-speed compressed airflow after thermal expansion affected the weighing module reading. Firstly,peeled weighing of the weighing module was performed. The reactor set at 500 ℃ was allowed to descend to the designated location, and 100, 200, 300, 400, and 500 mL/min of compressed air were introduced sequentially after the reading of the weighing module was stable. The results shown in Fig. 8 indicate that high-speed air blowing over the upper surface of the empty crucible does not affect the reading of the weighing module. In addition, a separate set of experiments demonstrates that the analytically pure Ca(OH)2 in the crucible are not blown off under a reactor temperature of 500 ℃ and an airflow of 500 mL/min.

Fig.8 Influence of high-speed compressed air on the reading of the weighing module
On the premise of not affecting the weighing module, a higher purge gas volume could enhance the mass transfer of the sample. For the kinetic studies of Ca(OH)2/CaO, a maximum gas flow of 100 mL/min[30] and a maximum superficial gas velocity of 0.05 m/s at 550 ℃[28] were adopted for the TGA. The purging gas volume of 500 mL/min, adopted for the fast-reaction TGA, provided better mass transfer conditions for samples than the volume and superficial gas velocity of the purge gas used for TGA.
Variable decoupling was used to clarify the influence of high-temperature air in the reactor and high-speed compressed air at the exit of the coil on the reading of the weighing module. The following multivariable thermal blank experiment was performed: peeled weighing was performed for the weighing module, and the reactor temperature was set to 500 ℃. A compressed air flow of 500 mL/min was introduced, and the reactor was allowed to descend to the designated location at different speeds (10, 20, 30, 40, and 50 mm/s). Because the temperature data collection frequency was set at 10 per second, the mass sampling frequency for this group of experiments was also set at 10 per second. The experimental results show similar trends in the readings of the weighing module. Because the slower reactor descending speed would separate the characteristic data points more obviously, the result of the reactor descending speed of 10 mm/s was also chosen, as shown in Fig. 9. The changing trend of the experimental data and the meaning of the characteristic points of the other reactor descending speed experiments are the same. The only difference is in the degree of separation of the characteristic data points. In conclusion, it is recommended to use a reactor descending speed of 50 mm/s to eliminate the influence of the weighing module, caused by the non-sample mass, in the shortest time.

Fig.9 Results of total factors blank experiment
2.5 Precision and accuracy of the fast-reaction TGA of the Ca(OH)2 decomposition reaction
The previous experiments investigating the influence of many variables on the reading of the weighing module and the related blank experiments were conducted without samples. To examine the precision and accuracy of the fast-reaction TGA technique, analytically pure Ca(OH)2 was used to conduct the experiments. The reactor temperature was set to 500 ℃, and 500 mL/min of compressed air was introduced. The descending speed of the reactor was set at 50 mm/s. When the reactor reached 500 ℃, a laboratory analytical balance (with an accuracy of 0.1 mg) was used to obtain the mass of the quartz crucible. Then 70.5 mg of the sample was added to the quartz crucible. The quartz crucible was placed on the quartz tray of the fast-reaction TGA experimental set-up, and the reactor was allowed to descend quickly to start the sample experiment. After the sample experiment was completed, a set of blank experiments was performed without the sample. Finally, the TG decomposition curve of the analytically pure Ca(OH)2 sample was obtained by subtracting the blank experimental mass data from the sample experimental mass data. The accuracy of the fast-reaction TGA technique was determined by comparing the sample mass before decomposition obtained from fast-reaction TGA with the sample mass obtained from the laboratory analytical balance before the experiment.
These experiments employs high-frequency mass data collection, and the data thus obtained exhibits slight fluctuations. Therefore, the data obtained by subtracting the blank experimental value from the sample experimental value also exhibits slight fluctuations. Even with a data collection frequency of one per second, the data obtained shows the reaction process well. This frequency enables stable data acquisition and transmission. Therefore, in the follow-up experiment, the data collection frequency of the weighing module is set to one per second to obtain better results.
Fig.10 shows the rapid decomposition curves of Ca(OH)2 obtained with the fast-reaction TGA. The remaining mass fraction of the sample obtained with the fast-reaction TGA is 78.47%, and that obtained with the TGA (heated to 500 ℃ at 20 ℃/min) is 77.66%. The deviation of the results between the two analyses is only 0.81%, indicating that the fast-reaction TGA technique is sufficiently precise and accurate.

Fig.10 Fast decomposition of Ca(OH)2 in the fast-reaction TGA
3 Conclusions
1) Fast-reaction TGA can provide a high heating rate for samples. The higher the reactor temperature, the greater the average heating rate and maximum instantaneous heating rate of the sample. When the reactor temperatures are 450, 500, 550, and 600 ℃, the average heating rates of the samples are 1.47, 1.73, 2.30, and 2.89 ℃/s, respectively, and the maximum instantaneous heating rates are 25.14, 30.47, 33.29, and 37.49 ℃/s, respectively.
2) The reactor descending speed of 50 mm/s eliminates the influence of non-sample variables in the shortest time. The descent of the high-temperature reactor has a regular effect on the reading of the weighing module, whereas the high-speed airflow does not.
3)Rapid thermal decomposition of Ca(OH)2 can be obtained through the fast-reaction TGA technique, and its accuracy is verified. The deviation in the material conversion obtained with the fast-reaction TGA technique from that obtained with the TGA technique is only 0.81%.
[1] MAHON H, O′CONNOR D, FRIEDRICH D, et al. A review of thermal energy storage technologies for seasonal loops [J]. Energy, 2022, 239: 122207.
[2] COSTA S, KENISARIN M. A review of metallic materials for lat-ent heat thermal energy storage: Thermophysical properties, applications, and challenges[J]. Renewable and Sustainable Energy Reviews, 2022, 154: 111812.
[3] GBENOU T, FOPAH-LELE A, WANG K. Recent status and prospects on thermochemical heat storage processes and applications [J]. Entropy, 2021, 23: 953.
[4] YUAN Y, LI Y, ZHAO J. Development on thermochemical energy storage based on CaO-based materials: A review [J]. Sustainability, 2018, 10: 2660.
[5] YUAN Y, LI Y, DUAN L, et al. CaO/Ca(OH)2 thermochemical heat storage of carbide slag from calcium looping cycles for CO2 capture [J]. Energy Conversion and Management, 2018, 174: 8-19.
[6] FENG Y, ZHANG M, DENG B, et al. A device and method for thermochemical energy storage/release using carbide slag:CN 112604611B[P]. 2022-03-29.
[7] SCHAUBE F, KOHZER A, SCHUTZ J, et al. De- and rehydra-tion of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage. Part A: Experimental results [J]. Chemical Engineering Research and Design, 2013, 91: 856-864.
[8] SCHMIDT M, SZCZUKOWSKI C, ROSSKOPF C, et al. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide [J]. Applied Thermal Engineering, 2014, 62: 553-559.
[9] YAN J, ZHAO C. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage [J]. Applied Energy, 2016, 175: 277-284.
[10] SCHMIDT M, GUTIERREZ A, LINDER M. Thermochemical energy storage with CaO/Ca(OH)2 :Experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor [J]. Applied Energy, 2017, 188: 672-681.
[11] SCHMIDT M, LINDER M. Power generation based on the Ca(OH)2/
CaO thermochemical storage system:Experimental investigation of discharge operation modes in lab scale and corresponding conceptual process design [J]. Applied Energy, 2017, 203: 594-607.
[12] YAN J, ZHAO C, XIA B, et al. The effect of dehydration temperatures on the performance of the CaO/Ca(OH)2 thermochemical heat storage system [J]. Energy, 2019, 186: 115837.
[13] WANG B, WANG Z, MA Y, et al. Heat transfer enhancement of indirect heat transfer reactors for Ca(OH)2/CaO thermochemical energy storage system [J]. Processes, 2021, 9: 1136.
[14] SCHAUBE F, KOCH L, WORNER A, et al. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage [J]. Thermochimica Acta, 2012, 538: 9-20.
[15] YAN J, ZHAO C. Thermodynamic and kinetic study of the dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping [J]. Chemical Engineering Science, 2015, 138: 86-92.
[16] LONG X, DAI L, LOU B, et al. The kinetics research of thermochemical energy storage system Ca(OH)2/CaO [J]. International Journal of Energy Research, 2017, 41: 1004-1013.
[17] HUANG C, XU M, HUAI X. Experimental investigation on thermodynamic and kinetic of calcium hydroxide dehydration with hexagonal boron nitride doping for thermochemical energy storage [J]. Chemical Engineering Science, 2019, 206: 518-526.
[18] LI Y, LI M, XU Z, et al. Dehydration kinetics and thermodynamics of ZrO(NO3)2-doped Ca(OH)2 for chemical heat storage [J]. Chemical Engineering Journal, 2020, 399: 125841.
[19] GUPTA A, ARMATIS P, SABHARWALL P, et al. Kinetics of Ca(OH)2 decomposition in pure Ca(OH)2 and Ca(OH)2-CaTiO3 composite pellets for application in thermochemical energy storage system [J]. Chemical Engineering Science, 2021, 246: 116986.
[20] PARDO P, ANXIONNAZ-MINVIELLE Z, ROUGE S, et al. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage [J]. Solar Energy, 2014, 107: 605-616.
[21] CRIADO Y, ALONSO M, ABANADES J, et al. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors [J]. Applied Thermal Engineering, 2014, 73: 1087-1094.
[22] CRIADO Y, HUILLE A, ROUGE S, et al. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications [J]. Chemical Engineering Journal, 2017, 313: 1194-1205.
[23] ROUGE S, CRIADO Y, SORIANO O, et al. Continuous CaO/Ca(OH)2 fluidized bed reactor for energy storage: First experimental results and reactor model validation [J]. Industrial & Engineering Chemistry Research, 2017, 56: 844-852.
[24] WUERTH M, BECKER M, OSTERMEIER P, et al. Developm-ent of a continuous fluidized bed reactor for thermochemical energy storage application [J]. Journal of Energy Resources Technology, 2019, 141: 070710.
[25] BIAN Z, LI Y, ZHANG C, et al. Heat release performance and evolution of CaO particles under fluidization for CaO/Ca(OH)2 thermochemical heat storage [J]. Process Safety and Environmental Protection, 2021, 155: 166-176.
[26] YIN J, FANG Y, ZHU X, et al. Kinetic study on CaCO3 decomposition via MFB-IR thermal analyzer [J]. Journal of Engineering Thermophysics, 2014, 35: 1216-1220.
[27] JIANG W, HAO W, LIU X, et al. Characteristics and kinetics of light calcination of magnesite in micro fluidized bed reaction analyzer [J]. CIESC Journal, 2019, 70: 2928-2937.
[28] CRIADO Y, ALONSO M, ABANADES J. Kinetics of the CaO/Ca(OH)2 hydration/dehydration reaction for thermochemical energy storage applications [J]. Industrial & Engineering Chemistry Research, 2014, 53: 12594-12601.
[29] ZENG X, WANG F, YU J, et al. Fundamentals and applications of micro fluidized bed reaction analysis [J]. Chemical Industry and Engineering Progress, 2016, 35: 1687-1697.
[30] IRABIEN A, VIGURI J, ORTIZ I. Thermal dehydration of calcium hydroxide. 1. Kinetic model and parameters [J]. Industrial & Engineering Chemistry Research, 1990, 29: 1599-1606.
快速反应热重分析仪的开发和基础特性

Mobile reading